Abstract
Glehnia littoralis is an important medicinal herb disjunctly distributed at sandy beaches in eastern Asia and western North America. The complete chloroplast genome sequence of G. littoralis is 147,552 bp in length and structurally divided into four distinct regions: two copies of inverted repeat of 18,365 bp separated by a large single copy (LSC) of 93,277 bp and a small single copy (SSC) of 17,545 bp. A total of 129 genes are annotated including 85 protein-coding genes, 36 tRNA gene, and eight rRNA genes. Phylogenetic relationship revealed that G. littoralis is closely related to Angelica dahurica in Apiaceae.
Glehnia littoralis Fr. Schmidt ex Miq., a member of carrot family (Apiaceae), is a typical coastal species distinctive distributed in eastern Asia and western North America disjunctly. Its dried roots and rhizomes, generally called Bei-Sha-Shen, are important traditional Chinese medicine for treating lung diseases. Wild G. littoralis is rare and Endangered at present because of habitat loss and over-exploitation. The population in Jiangsu province was even local extinction (Song et al. Citation2013). It has been listed as a second-level protected species by Chinese government and a Critically Endangered species (CR) in China Biodiversity Red List—Higher Plants. However, there is no comprehensive genetic researches were conducted for G. littoralis due to lack of genomic resources until now. Therefore, we assembled the complete chloroplast (cp) genome of G. littoralis based on Illumina paired-end sequencing. Our study will provide a valuable plastid genomic resource for population genetics studies.
The individuals of G. littoralis were collected from Xichong Beach, Shenzhen (22°28′39.11″ N, 114°31′54.72″ E). The voucher specimen has been deposited in the Herbarium of Institute of Botany, Jiangsu Province and Chinese Academy of Sciences (NAS). The total genomic DNA was extracted from fresh leaves using Plant Genomic DNA Kit DP305 (Tiangen, Beijing) and sequenced on Illumina Hiseq TM 4000 platform (San Diego, CA). The raw data were assembled after filtering out low-quality sequences according to the protocol of Hahn et al. (Citation2013). The complete cp genome was annotated with DOGMA (Wyman et al. Citation2004) and adjusted start and stop codons manually. The circular cp genome map was drawn using OGDRAW (Lohse et al. Citation2013).
The complete cp genome sequence of G. littoralis (MH142518) was 147,552 bp in length. It harbored a typical quadripartite and circular structure including a large single copy (LSC) of 93,277 bp and a small single copy (SSC) of 17,545 bp separated by a pair of inverted repeat (IRa and IRb) of 18,365 bp. In the chloroplast genome, 114 unique genes were identified, containing 80 protein-coding genes, 30 transfer RNA (tRNA), and four ribosomal RNA (rRNA). Sixteen genes contained one intron, including 10 protein-coding genes, and six tRNA. Ycf3 and clpP had two introns. The GC content of whole cp genome was 37.5%.
To explore the divergence hotspot regions within G. littoralis, the cp genome sequence was compared to another available G. littoralis (KT153022) (Lee et al. Citation2015) comprehensively at the genome scale. We detected 47 mutations (26 SNPs and 21 indels), 38 in the LSC region, five in the SSC region, and four in the IR regions. All these sites would provide potential molecular markers for further conservation genetics and phylogeographic studies of G. littoralis.
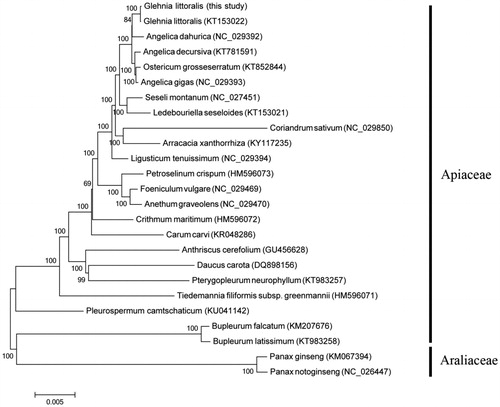
An alignment was generated in Geneious 10 (Kearse et al. Citation2012) using the MAFFT plugin (Katoh and Standley Citation2013) to reconstruct molecular phylogenetic tree within Apiaceae with the Neighbor-Joining (NJ) method that employed in MEGA 6 (Tamura et al. Citation2013). Two species in Araliaceae were chosen as outgroups. As a result, individuals of G. littoralis formed a well-supported monophyletic clade closely related to Angelica dahurica ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads – a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649.
- Lee SC, Oh Lee H, Kim K, Kim S, Yang TJ. 2015. The complete chloroplast genome sequence of the medicinal plant Glehnia littoralis F. Schmidt ex Miq. (Apiaceae). Mitochondrial DNA A DNA Mapp Seq Anal. 27:3674.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. OrganellarGenomeDRAW – a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:W575–W581.
- Song C, Wu B, Hu J, Dong Z, Liu Q. 2013. Existence status of Glehnia littoralis and causes of extinction in Jiangsu Province. Chinese Wild Plant Resour. 32:56–57. 69.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.