Abstract
Piper laetispicum is dioecious climbers woody with 10 m tall, which is an endemic species in China. It grows on trees or rocks in forests (100–600 m) in Guangdong and Hainan Province of China. Here, we report and characterize the complete plastid genome sequence of P. laetispicum in an effort to provide genomic resources useful for promoting its conservation. The complete plastome is 161,721 bp in length and contains the typical structure and gene content of angiosperm plastome, including two inverted repeat (IR) regions of 27,125 bp, a large single copy (LSC) region of 89,224 bp and a small single copy (SSC) region of 18,247 bp. The plastome contains 114 genes, consisting of 80 unique protein-coding genes, 30 unique tRNA gene, and four unique rRNA genes. The overall A/T content in the plastome of P. laetispicum is 61.70%. The complete plastome sequence of P. laetispicum will provide a useful resource for the conservation genetics of this species as well as for the phylogenetic studies for Piper.
Piper laetispicum C. DC (Piperaceae, Piperales) is dioecious climbers woody with 10 m tall, which is an endemic species in China. It grows on trees or rocks in forests (100–600 m) in Guangdong and Hanan Province of China (Cheng et al. Citation1999). Consequently, the genetic and genomic information is urgently needed to promote its systematics research and the development of conservation value of P. laetispicum. Here, we report and characterize the complete plastome of P. laetispicum (GenBank accession number: MH678665, this study). This is the first report of a complete plastome for the P. laetispicum.
In this study, P. laetispicum was sampled from Diaoluo Mountain (18.67°N, 109.88°E), which is a National Nature Reserve of Hainan, China. A voucher specimen (H.F. Wang, B36) was deposited in the Herbarium of the Institute of Tropical Agriculture and Forestry (HUTB), Hainan University, Haikou, China.
The modified cetyltrimethyl ammonium bromide (CTAB) protocol of Doyle and Doyle (Citation1987) was used to extract genomic DNA from dry leaf tissues. The genomic DNA of each sample was quantified and analysed with Agilent 2100 BioAnalyzer (Palo Alto, CA). Samples of yield at least 0.8 μg DNA were selected for subsequent libraries construction and de novo sequencing. Genomic DNA of selected samples were used to build the paired-end libraries with 200–400 bp insert size. Libraries were sequenced using BGISEQ-500 platform at BGI (Shenzhen, China) and produced about 8 Gb high quality per sample with 100 bp paired-end reads. Raw reads were trimmed using SOAPfilter_v2.2 with the following criteria (1) reads with >10% base of N; (2) reads with >40% of low quality (value ≤10); (3) reads contaminated by adaptor and produced by PCR duplication. Around 6 Gb clean data were assembled against the plastome of Piper kadsura (KT223569.1) (Lee et al. Citation2015) using MITO bim v1.8 (Hahn et al. Citation2013).
The plastome was annotated using Geneious R8.0.2 (Biomatters Ltd., Auckland, New Zealand) against the plastome of Piper kadsura (KT223569.1). The annotation was corrected with DOGMA (Wyman et al. Citation2004).
The plastome of P. laetispicum was found to possess a total length 161,721 bp with the typical quadripartite structure of angiosperms, containing two inverted repeats (IRs) of 27,125 bp, a large single copy (LSC) region of 89,224 bp and a small single copy (SSC) region of 18,247 bp. The plastome contains 114 genes, consisting of 80 unique protein-coding genes, 30 unique tRNA genes, and four unique rRNA genes. Among these genes, 15 genes (trnK-UUU, rps16, trnG-UCC, atpF, rpoC1,trnL-UAA, trnV-UAC, trnI-GAU, trnA-UGC, rpl12, petB, petD, rpl16, ndhB, ndhA) possessed a single intron and three genes (ycf3, clpP, rps12) had two introns. The gene rps12 was found to be trans-spliced, as is typical of angiosperms. The overall A/T content in the plastome of P. laetispicum is 61.70%, in which the corresponding value of the LSC, SSC, and IR region was 63.20%, 67.90%, and 57.10%, respectively.
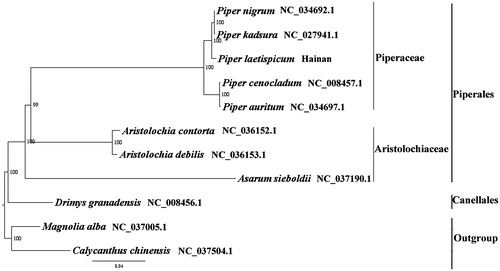
We used RAxML (Stamatakis Citation2006) with 1000 bootstraps under the GTRGAMMAI substitution model to reconstruct a maximum likelihood (ML) phylogeny of seven published complete plastome of Piperales and one published complete plastome of Canellales, using Magnolia alba (Magnoliaceae, Magnoliales) and Calycanthus chinensis (Calycanthaceae, Laurales) as outgroups. The phylogenetic analysis indicated that P. laetispicum is closer to P. kadsura with maximum bootstrap support (). Most nodes in the plastome ML trees were strongly supported. The complete plastome sequence of P. laetispicum will provide a useful resource for the conservation genetics of this species as well as for the phylogenetic studies for Piper.
Figure 1. The best ML phylogeny recovered from 11 complete plastome sequences by RAxML. Accession numbers: Piper laetispicum (MH678665, this study), Piper nigrum NC_034692.1, Piper kadsura NC_027941.1, Piper cenocladum NC_008457.1, Piper auritum NC_034697.1, Aristolochia contorta NC_036152.1, Aristolochia debilis NC_036153.1, Asarum sieboldii NC_037190.1, Drimys granadensis NC_008456.1; outgroup: Magnolia alba NC_037005.1, Calycanthus chinensis NC_037504.1.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cheng Y, Tseng Y-C, Xia N, Gilbert MG. 1999. Flora of China, vol. 4. Beijing: Science Press; p. 110–131.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads-a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129.
- Lee JH, Choi IS, Choi BH, Yang S, Choi G. 2015. The complete plastid genome of Piper kadsura (Piperaceae), an East Asian woody vine. Mitochondrial DNA A DNA Mapp Seq Anal. 27:3555.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
