Abstract
The Hyporhamphus dussumieri is a kind of minor commercial fishes, usually marketed fresh and dried salted. However, to date, there is a limited genetic resource available for this species. In this study, we assembled the whole mitochondrial genome of this species yielding a 16,542 bp circular assembly composed of the typical vertebrate mitochondrial features. It contains 13 protein-coding genes, two rRNA genes, 22 tRNA genes, a putative control region, and one origin of replication on the light-strand. The overall base composition includes C(28.3%), A(28.9%), T(27.1%), and G(15.7%). Moreover, the 13 PCGs encode 3800 amino acids in total. The result of the phylogenetic tree supports H. dussumieri has a closest relationship with Hemiramphus far.
The Dussumier’s halfbeak (Hyporhamphus dussumieri) lives in reefs and shallow lagoons. They live solitary or in groups, and are often seen circling snorkelers, just under the surface of the water (Fricke et al. Citation2011), most common around islands and marketed fresh and dried salted (Carpenter and Carpenter Citation2002). Genetic resources for this species are presently lacking. In this study, we described the complete mitochondrial genome of H. dussumieri and explored the phylogenetic relationship within Beloniformes, to gain its molecular information and thus contribute to facilitate future studies on population genetic structure and phylogenetic relationships.
Here, we assembled the mitogenome of H. dussumieri by Codoncode Aligner, which was sampled from Tsankiang, China (21°16'25"N; 110°21'16"E) and stored in laboratory of Zhejiang Ocean University with accession number 20150826dsxz22. Similar to the typical mitogenome of vertebrates (Zhu et al. Citation2018a, Citation2018c), the mitogenome of H. dussumieri is a closed double-stranded circular molecule of 16,542 nucleotides (GenBank accession no. MH734933), within the range of other teleost mitogenomes. The complete mitochondrial genome contains 13 protein-coding genes (PCGs), two ribosomal RNA genes (12S rRNA and 16S rRNA), 22 transfer RNAs (tRNA) genes, and a putative control region (CR) and one origin of replication on the light-strand (OL). The overall base composition is 28.9% A, 28.3% C, 27.1% T, and 15.7% G, respectively, with a slight AT bias (56.0%). Most mitochondrial genes of H. dussumieri were encoded on the H-strand, with only ND6 and eight tRNA (Gln, Ala, Asn, Cys, Tyr, Ser-UCN, Glu, and Pro) genes encoded on the L-strand. 13PCGs genes encode 3800 amino acids in total, all of them use the initiation codon ATG except COI starts with GTG, which is quite common in vertebrate mtDNA (Miya et al. Citation2001; Zhu et al. Citation2018b). Most of them have TAA or TAG as the stop codon, whereas three protein-coding genes (COII, ND4, Cytb) ended with a single T. The lengths of 12S rRNA located between tRNAPhe and tRNAVal and 16S rRNA located between tRNAVal and tRNALeu were 946 bp and 1691 bp, respectively. The origin of light-strand replication is located in a cluster of five tRNA genes (WANCY), which has the potential to fold into a stable stem-loop secondary structure, with a stem formed by 13 paired nucleotides and a loop of 13 nucleotides; the CR is determined to be 876 bp, which is located between the tRNA-Pro and tRNA-Phe genes, and three typical domains are observed, including the TAS, central CSB and CSB, which is identical to that in other teleostean mitogenomes (Zhang et al. Citation2013).
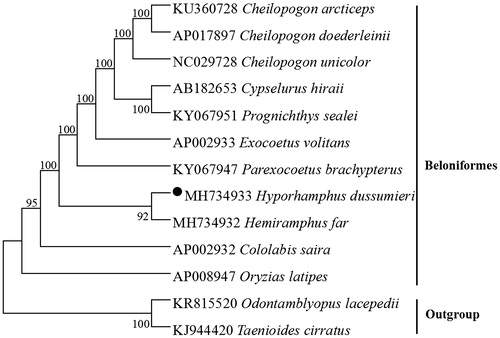
The phylogenetic tree based on the neighbour-joining method was constructed to provide relationship within Beloniformes. The result of the present study supports H. dussumieri has a closest relationship with Hemiramphus far, highly supported by a bootstrap probability of 92% (), this characteristic likely contributes to the high population genetic structuring typically observed in species belonging to Hemiramphidae (Meisner and Burns Citation1997; Tibbetts et al. Citation2007).
Figure 1. Neighbour-joining (NJ) tree of 11 Beloniformes species based on 12 PCGs encoded by the heavy strand. The bootstrap values are based on 1000 resamplings. The number at each node is the bootstrap probability. The number before the species name is the GenBank accession number. The genome sequence in this study is labeled with a black spot.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Carpenter KE, Carpenter KE. 2002. The living marine resources of the Western Central Atlantic. Volume 2: bony fishes Part 1 (Acipenseridae to Grammatidae). FAO Species Identification Field Guide for Fishery Purposes (FAO) Special Publication – American Society of Ichthyologists and Herpetologists (USA). 2005:359–363.
- Fricke R, Kulbicki M, Wantiez L. 2011. Checklist of the fishes of New Caledonia, and their distribution in the Southwest Pacific Ocean (Pisces). Folia Pharmacol Jpn. 96:31–39.
- Meisner AD, Burns JR. 1997. Viviparity in the halfbeak genera Dermogenys and Nomorhamphus (Teleostei: Hemiramphidae). J Morphol. 234:295.
- Miya M, Kawaguchi A, Nishida M. 2001. Mitogenomic exploration of higher teleostean phylogenies: a case study for moderate-scale evolutionary genomics with 38 newly determined complete mitochondrial DNA sequences. Mol Biol Evol. 18:1993–2009.
- Tibbetts I, Isaac R, Kreiter P. 2007. Functional and phylogenetic implications of the vesicular swimbladder of Hemiramphus and Oxyporhamphus convexus (Beloniformes: Teleostei). Copeia. 2007:808–817.
- Zhang H, Zhang Y, Zhang X, Song N, Gao T. 2013. Special structure of mitochondrial DNA control region and phylogenetic relationship among individuals of the black rockfish, Sebastes schlegelii. Mitochondrial DNA. 24:151–157.
- Zhu K, Gong L, Jiang L, Liu L, Lü Z, Liu B-j. 2018a. Phylogenetic analysis of the complete mitochondrial genome of Anguilla japonica (Anguilliformes, Anguillidae). Mitochondrial DNA B. 3:536–537.
- Zhu K, Gong L, Lü Z, Liu L, Jiang L, Liu B. 2018b. The complete mitochondrial genome of Chaetodon octofasciatus (Perciformes: Chaetodontidae) and phylogenetic studies of Percoidea. Mitochondrial DNA B. 3:531–532.
- Zhu K, Lü Z, Liu L, Gong L, Liu B. 2018c. The complete mitochondrial genome of Trachidermus fasciatus (Scorpaeniformes: Cottidae) and phylogenetic studies of Cottidae. Mitochondrial DNA B. 3:301–302.
