Abstract
The mitochondrial genome of Epeorus herklotsi (Ephemeroptera: Heptageniidae) from Longquan, Zhejiang province, China was a circular molecule of 15,499 bp in length, containing 13 protein-coding genes, two ribosomal RNAs, 23 transfer RNAs, and a control region. The A + T content of the overall base composition of H-strand is 65.7%. All of the 13 protein-coding genes were begun with ATN as start codon except ND5 using GTG. ATP6, ATP8, COI, COIII, ND1, ND2, ND4L, and ND6 genes were terminated with TAA as stop codon, Cyt b ended with TAG and the other three protein-coding genes end with an incomplete stop codon (TA or T). In BI phylogenetic trees, the monophyly of the families Caenidae, Heptageniidae, Isonychiidae and Viemamellidae, and the genus Epeorus were strongly supported. Epeorus is a sister clade to Parafronurus, then cluster with Paegniodes.
The heptageniid mayflies Epeorus herklotsi is widely distributed throughout the rivers of China. For the convenience of identifying this species, we sequenced the mitochondrial genome of E. herklotsi. In Ephemeroptera, gene rearrangements were found in six sequences of twenty-three known mayfly mitochondrial genomes with IMQM tRNA cluster found in Heptageniidae (Zhang et al. Citation2008; Li et al. Citation2014; Tang et al. Citation2014; Gao et al. Citation2018). The phylogenetic relationships of Ephemeroptera still exist disputes in morphological and molecular methods (Kristensen Citation1981; Ogden and Whiting Citation2003; Zhang et al. Citation2008; Simon and Hadrys Citation2013; Li et al. Citation2014; Misof et al. Citation2014; Cai et al. Citation2018; Ye et al. Citation2018). Hence, we used the mitochondrial genome of E. herklotsi to analyse the characteristics of mitochondrial gene arrangement and to discuss the phylogenetic relationships within Ephemeroptera.
The samples of E. herklotsi from Longquan city, Zhejiang province, China were stored at −70 °C in our laboratory at Shaoxing People’s hospital. Some DNA fragments were amplified using highly conserved primers (Zhang et al. Citation2008). After obtaining most part of the mitogenome, we designed species-specific primers for the remaining part with reference to previously determined sequences. All polymerase chain reactions (PCRs) were carried out using a MyCyclere Thermal Cycler (Bio-Rad, Hercules, CA). TaKaRa Ex-Taq and LA-Taq Kits (Takara Biomedical, Dalian, China) were used for normal and long PCRs.
The complete mtDNA is 15,499 bp in length and contains 13 protein-coding genes, two ribosomal RNAs, 23 transfer RNAs genes, and noncoding regions. The overall base composition of H-strand is as follows: T (33%), C (21.3%), A (32.7%), G (13%), and the A + T content (65.7%). All of the 13 protein-coding genes were begun with ATN as start codon except ND5 using GTG. ATP6, ATP8, COI, COIII, ND1, ND2, ND4L, ND6 genes were terminated with TAA as stop codon, Cyt b ended with TAG and the other protein-coding genes ended with an incomplete stop codon (TA or T). An IMQM tRNA cluster at the upstream of ND2 gene was found, which was consistent with P. youi (Zhang et al. Citation2008) and three species of Epeorus (Tang et al. Citation2014; Gao et al. Citation2018), while it was different to Paegniodes cupulatus with the typical IQM tRNA cluster (Zhou et al. Citation2016).
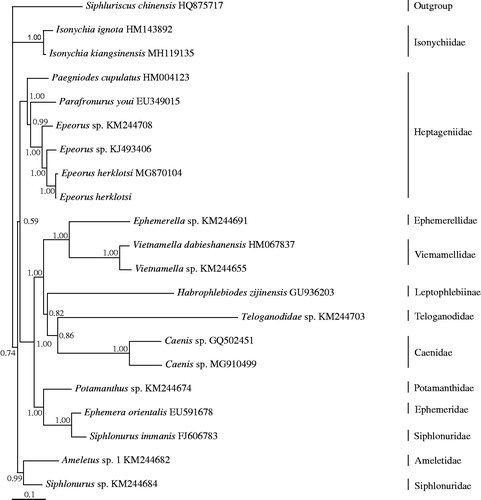
Bayesian inference (BI) tree was constructed using the 13 PCGs from 21 species using S. chinensis (Li et al. Citation2014) as outgroup (). BI analysis was performed by MrBayes version 3.1.2 (Huelsenbeck and Ronquist Citation2001). In the results, the monophyly of the families Caenidae, Heptageniidae, Isonychiidae, Viemamellidae, and the genus Epeorus was strongly supported. Isonychiidae was the basal clade to Ephemeroptera excluding Siphluriscidae. The monophyly of the family Siphlonuridae was not supported in our results, because Siphlonurus sp. (KM244684) and S. immanis (FJ606783) were cluster with Ameletus sp. 1 (KM244682) and Ephemera orientalis, respectively. Within the Heptageniidae clade, Epeorus was a sister clade to Parafronurus, then (Epeorus + Parafronurus) cluster with Paegniodes. Two species of Epeorus herklotsi were cluster together.
Figure 1. Phylogenetic tree of the relationships among 21 species of Ephemeroptera, including Epeorus herklotsi from Longquan, Zhejiang province, China based on the nucleotide dataset of the 13 mitochondrial protein-coding genes. The Bayesian posterior probability values are indicated above nodes. The GenBank numbers of all species are shown in the figure.
Nucleotide sequence accession number
The complete mitochondrial genome of E. herklotsi has been assigned deposited to GenBank with the following accession number MH752075.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Cai YY, Gao YJ, Zhang LP, Yu DN, Storey KB, Zhang JY. 2018. The mitochondrial genome of Caenis sp. (Ephemeroptera: Caenidae) and the phylogeny of Ephemeroptera in Pterygota. Mitochondrial DNA B. 3:577–579.
- Gao XY, Zhang SS, Zhang LP, Yu DN, Zhang JY, Cheng HY. 2018. The complete mitochondrial genome of Epeorus herklotsi (Ephemeroptera: Heptageniidae) and its phylogeny. Mitochondrial DNA B. 3:303–304.
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755.
- Kristensen NP. 1981. Phylogeny of insect orders. Annu Rev Entomol. 26:135–157.
- Li D, Qin JC, Zhou CF. 2014. The phylogeny of Ephemeroptera in Pterygota revealed by the mitochondrial genome of Siphluriscus chinensis (Hexapoda: Insecta). Gene. 545:132–140.
- Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, Frandsen PB, Ware J, Flouri T, Beutel RG, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science. 346:763–767.
- Ogden TH, Whiting MF. 2003. The problem with “the Paleoptera problem” sense and sensitivity. Cladistics. 19:432–442.
- Simon S, Hadrys H. 2013. A comparative analysis of complete mitochondrial genomes among Hexapoda. Mol Phylogenet Evol. 69:393–403.
- Tang M, Tan M, Meng G, Yang S, Su X, Liu S, Song W, Li Y, Wu Q, Zhang A, et al. 2014. Multiplex sequencing of pooled mitochondrial genomes-a crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 42:e166.
- Ye QM, Zhang SS, Cai YY, Storey KB, Yu DN, Zhang JY. 2018. The complete mitochondrial genome of Isonychia kiangsinensis (Ephemeroptera: Isonychiidae). Mitochondrial DNA B. 3:541–542.
- Zhang JY, Zhou CF, Gai YH, Song DX, Zhou KY. 2008. The complete mitochondrial genome of Parafronurus youi (Insecta: Ephemeroptera) and phylogenetic position of the Ephemeroptera. Gene. 424:18–24.
- Zhou D, Wang YY, Sun JZ, Han YK, Zhou CF. 2016. The complete mitochondrial genome of Paegniodes cupulatus (Ephemeroptera: Heptageniidae). Mitochondrial DNA A. 27:925–926.
