Abstract
The complete mitochondrial genome of Fimbriaria fasciolaris is reported for the first time in this study. Its entire mitogenome is 13,543 bp in length, contained 12 protein-coding genes, 2 ribosomal RNA genes, 22 transfer RNA genes, and 2 non-coding regions. The arrangement of the twelve PCGs was identical to that of most cestode mtDNA sequence. Among all the cestodes mitochondrial genomes consulted from GenBank database, there existed a tiny difference in the order of trnY, trnL1, trnL2, and trnS2. The phylogenetic analysis by Bayesian inference method shows that F. fasciolaris of this study was confirmed to be a member of family Hymenolepididae.
Fimbriaria fasciolaris (Pallas, 1781) is a parasitic of the class Cestoda, phylum Platyhelminthes. It infects the anterior intestine of Anseriformes, including duck and other Wild birds. F. fasciolaris is widely distributed in Europe, North America, Africa, Asia, Central America, South America. The cestode is recorded in some species of ducks with a prevalence of 55.6% and a mean intensity of infection of 60.8%, being, so far, one of the highest percentages registered (Muniz-Pereira and Amato Citation1998). Bring huge economic loss to poultry farming industry.
Adult cestode was obtained from the small intestine of an infected domestic duck from a Farm (46.0390567552 N, 124.8105741947 E), Datong region of Daqing, Heilongjiang Province, China, on 23 August 2017. The individual cestode was stored in the Department of Parasitology, Heilongjiang Bayi Agricultural University (specimen no. BYNKPL-170823), and DNA was extracted by TIANamp Genomic DNA Kit (TIANGEN, Beijing, China), and stored at –20 °C until use. Primers were designed for polymerase chain reaction (PCR) amplification and sequencing on the basis of the mitogenome sequence of Drepanidotaenia lanceolata (GenBank accession No. KR817910) (Gao et al. Citation2017).
The entire F. fasciolaris mt genome was a typical circular DNA molecule with 13,543 bp in size (GenBank accession number MH551238). There were twelve PCGs (cox1-3, nad1-6, nad4L, atp6 and cytb), twenty-two transfer RNA (tRNA) genes, two ribosomal RNA (rRNA) genes and two non-coding regions including in the mtDNA sequence of F. fasciolaris. Arrangement of the twelve PCGs was identical to that of most cestode mtDNA sequence (Gao et al. Citation2017). Among all the cestodes mitochondrial genomes consulted from GenBank database, there existed a tiny difference in the order of trnY, trnL1, trnL2, and trnS2. Cestodes of family Taeniidae and Diphyllobothriidae which commonly infect mammals share the same arrangement of trnY-trnL1-trnS2-trnL2 (Yamasaki et al. Citation2012). Fimbriaria fasciolaris in this study, other cestodes of family Hymenolepididae, Paruterinidae, Dilepididae and Anoplocephdidae which mostly infect mammals and avian are the same in the arrangement of trnY-trn S2-trn L1-trnL2 (Guo Citation2016). Cestodes of family Bothriocephalidae which mainly infect fish have an arrangement of trnL1-trnL2-trnY-trnS2 (Brabec et al. Citation2016).
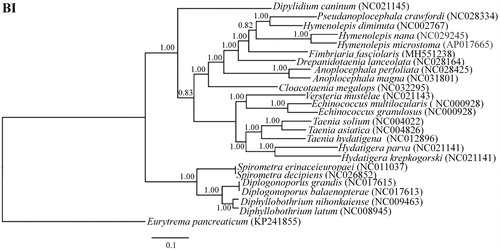
Based on the concatenated amino acid sequence dataset (12 protein-coding genes), phylogenetic analyses were performed using Bayesian inference (BI). The result showed that phylogenetic tree claded into three distinct groups with high statistical support (). Family Taeniidae (includes genus Taenia, genus Hydatigera, genus Echinococcus and genus Versteria) formed a clade, family Hymenolepididae (includes genus Hymenolepis, genus Pseudanoplocephala, genus Drepanidotaenia, genus Dicranotaenia, and genus Cloacotaenia) along with family Anoplocephalidae and family Dipylidiidae formed a clade, family Diphyllobothriidae (includes genus Diplogonoporus, genus Diphyllobothrium, and genus Spirometra) formed a clade. F. fasciolaris of this study was confirmed to be a member of family Hymenolepididae, and it was closer to the Hymenolepis diminuta and Penion crawfordi than D. lanceolata which could also infect again. Therefore, the conserved mtDNA is the valid tool to determine the taxonomic status of cestodes in family Hymenolepididae, anticipating more mtDNA data for phylogenetic studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brabec J, Kuchta R, Scholz T, Littlewood DT. 2016. Paralogues of nuclear ribosomal genes conceal phylogenetic signals within the invasive Asian fish cestode lineage: evidence from next generation sequencing data. Int J Parasitol. 46:555–562.
- Gao JF, Hou MR, Cui YC, Wang LK, Wang CR. 2017. The complete mitochondrial genome sequence of Drepanidotaenia lanceolata (Cyclophyllidea: Hymenolepididae). Mitochondrial DNA Part A. 28:317–318.
- Guo A. 2016. The complete mitochondrial genome of the tapeworm Cladotaenia vulturi (Cestoda: Paruterinidae): gene arrangement and phylogenetic relationships with other cestodes. Parasit Vectors. 9:475.
- Muniz-Pereira LC, Amato SB. 1998. Fimbriaria fasciolaris and Cloacotaenia megalops (Eucestoda, Hymenolepididae), cestodes from Brazilian waterfowl. Mem Inst Oswaldo Cruz. 93:767.
- Yamasaki H, Ohmae H, Kuramochi T. 2012. Complete mitochondrial genomes of Diplogonoporus balaenopterae and Diplogonoporus grandis (Cestoda: Diphyllobothriidae) and clarification of their taxonomic relationships. Parasitol Int. 61:260–266.