Abstract
Trichosanthes kirilowii Maxim. is an essential traditional Chinese medicine used for various diseases. The complete chloroplast genome sequence of T. kirilowii has been determined in this study. The total genome size is 157,481 bp in length and contains a pair of inverted repeats (IRs) of 26,268 bp, which were separated by large single copy (LSC) and small single copy (SSC) of 86,478 bp and 18,467 bp, respectively. A total of 130 genes were predicted including 85 protein-coding genes, eight rRNA genes and 37 tRNA genes. Further, phylogenetic analysis confirmed that T. kirilowii belongs to the family Cucurbitaceae. The complete chloroplast genome of T. kirilowii would play a significant role in the development of molecular markers in plant phylogenetic and population genetic studies.
Trichosanthes kirilowii Maxim. commonly known as ‘snake gourd’, is an essential traditional Chinese medicine to treat thoracic obstruction, angina, cardiac failure, myocardial infarction, pulmonary heart disease, some cerebral ischaemic diseases, etc. (Yu et al. Citation2018). The species was origin and diversified in Asia (Schaefer et al. Citation2009; Zhou et al. Citation2015). The seeds, pericarps and fruits of T. kirilowii contained approximately 162 compounds with pharmacological activities (Yu et al. Citation2018). Therefore, the species have immense medical and economic value. However, the precious medicine was often suffered from substitution and adulteration of its closely related species, such as Trichosanthes hupehensis, Siraitia grosvenorii, and Momordica cochinchinensis (Lv and Yu Citation2017), which resulting in decreasing in the efficacy of drug or being lethal in some cases(Ganie et al. Citation2015). Chloroplast genome is a molecular resource for developing DNA barcoding and marker for plant identification (Techen et al. Citation2014). However, to the best of our knowledge, there are no reports that the chloroplast genome of T. kirilowii was taken as a molecular resource. Thus, in this study, our aim to sequence the chloroplast completes genome of T. kirilowii with the hope of promoting the studies on the species identification, germplasm exploration and phylogenetic relationships.
Genomic DNA was extracted from fresh leaves of T. kirilowii plant cultivated in the illumination incubator at Jiangxi Agricultural University (28°45′53″N, 115°49′41″), Nanchang, China. About 15μg extracted DNA was sent to BioMarker (Beijing, China) for library construction and genome sequencing on the HiSeq X Ten Platform. After sequencing and base quality control, a total of 1 Gb of sequence data in fastq format was obtained. The draft genome sequence was assembled by using the Plasmidspades.py in SPAdes v3.12.0 (Bankevich et al. Citation2012), BlastN v2.7.1, and Gapcloser v1.12-r6. Contigs representing the chloroplast genome were then retrieved, ordered and incorporated into a single draft sequence by comparison with the chloroplast genome of Citrullus colocynthis (NC_035727.1) using BlastN. The gaps in the chloroplast single draft sequence were closed by using GapCloser and the complete genome was confirmed and manually corrected by PE read mapping. Finally, the complete genome sequence was annotated using GeSeq (Tillich et al. Citation2017) and manually corrected by visual inspection using IGV (Robinson et al. Citation2011).
A typical chloroplast genome consists of four distinct regions, a large and a small single copy region (LSC and SSC, respectively) separated by two inverted repeat regions (IRa and IRb). The complete chloroplast genome of T. kirilowii (accession no. MK036046) is 157,481 bp in length with 37.64% GC contents, and exhibits a typical quadripartite structure, consisting of a pair of IRs (26,268 bp) separated by the LSC (86,478 bp) and SSC (18,467 bp) regions. There is a total of 130 genes, including 85 protein-coding genes, eight rRNA genes and 37 tRNA genes; six of the protein-coding genes, six of the tRNA genes and four rRNA genes are duplicated within the IRs.
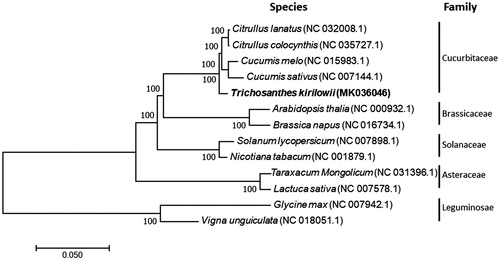
To determine the phylogenetic position of T. kirilowii, a phylogenetic tree included other 12 representative species was constructed by Neighbor-Joining method using the program MAFFT v7.407 (Nakamura et al. Citation2018) and MEGA v10.0.4 (Kumar et al. Citation2018). The results showed that T. kirilowii was clustered into the family Cucurbitaceae and closer to Citrullus and Cucumis, other species in different family were also well clustered into their corresponding clades ().
Figure 1. Phylogenetic tree showing relationship between Trichosanthes kirilowii Maxim. and other 12 species belonging to different families. Phylogenetic tree was constructed based on the complete chloroplast genomes using neighbour-joining (NJ) with 1000 bootstrap replicates. Numbers in each the node indicated the bootstrap support values.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol Comput Biol. 19:455–477.
- Ganie SH, Upadhyay P, Das S, Prasad Sharma M. 2015. Authentication of medicinal plants by DNA markers. Plant Gene. 4:83–99.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Lv Z, Yu J. 2017. Molecular identification of trichosanthes kirilowii and its adulterants using ITS2 sequence fragments. J Southwest Univ (Natural Science Edition) 39:1–6.
- Nakamura T, Yamada KD, Tomii K, Katoh K. 2018. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics. 34:2490–2492.
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol. 29:24–26.
- Schaefer H, Heibl C, Renner SS. 2009. Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Roy Soc Lond B Bio. 276:843–851.
- Techen N, Parveen I, Pan Z, Khan IA. 2014. DNA barcoding of medicinal plant material for identification. Curr Opin Biotechnol. 25:103–110.
- Tillich M, Lehwark P, Pellizzer T, Ulbrichtjones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45:W6–W11.
- Yu X, Tang L, Wu H, Zhang X, Luo H, Guo R, Xu M, Yang H, Fan J, Wang Z, et al. 2018. Trichosanthis Fructus: botany, traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 224:177–194.
- Zhou T, Huang L, Jiang W. 2015. On the evolution and distribution of the Trichosanthes. J Plant Sci. 33:414–423.
