Abstract
The pale summer sedge caddisfly, Limnephilus hyalinus Hagen, 1861 (Limnephilidae, the Northern Caddisflies), is widespread in North America. Genome skimming by Illumina sequencing allowed assembly of a complete 15,168 bp circular mitogenome from L. hyalinus consisting of 78.0% AT nucleotides, 22 tRNAs, 13 protein-coding genes, two rRNAs and a control region in the ancestral insect gene order. Limnephilus hyalinus COX1 features an atypical CGA start codon while ATP8, NAD1, NAD5, and NAD6 exhibit incomplete stop codons. The mtTERM binding site is conserved between the Trichoptera and the Lepidoptera. Phylogenetic reconstruction reveals a monophyletic Order Trichoptera, Family Limnephilidae, and genus Limnephilus.
The Living Prairie Mitogenomics Consortium is constructing a library of arthropod mitogenomes for improved DNA-based species identification and phylogenetics (Living Prairie Mitogenomics Consortium Citation2017, Citation2018; Marcus Citation2018) through an inquiry learning exercise in an undergraduate course (Marcus et al. Citation2010). Students who successfully analysed the class data (which were further curated by the instructor) belong to our consortium.
On 14–15 August 2015, we collected night-flying insects at the Living Prairie Museum (GPS 49.889607 N, –97.270487 W) using a USDA blacklight trap (Winter Citation2000). One adult specimen of the pale summer sedge caddisfly, Limnephilus hyalinus Hagen, 1861 (Insecta: Trichoptera: Limnephilidae, project specimen number 2015.08.14.076), was trapped, pinned, identified (Ruiter Citation1995; Schwiebert Citation2007), and deposited in the Wallis Roughley Museum of Entomology at the University of Manitoba (voucher JBWM0379996).
Limnephilus is a large genus with over 150 species that are frequently abundant in high latitude and high altitude lacustrine habitats (Ruiter Citation1995; Schwiebert Citation2007; McCullagh et al. Citation2015). Limnephilus hyalinus occurs from Alaska to Colorado, and from British Columbia to New England in lakes and rivers, with adults emerging July-September (Schwiebert Citation2007; Houghton Citation2012). Here, we describe the first complete mitogenome for a New World Limnephilus from L. hyalinus.
DNA was prepared (McCullagh and Marcus Citation2015) and sequenced by Illumina MiSeq (San Diego, CA) (Peters and Marcus Citation2017). We assembled the mitogenome of L. hyalinus (GenBank MK077681) with Geneious 10.1.2 from 7,020,834 paired 75 bp reads using an Anabolia bimaculata (Trichoptera: Limnephilidae) reference mitogenome (MF680449) (Peirson and Marcus Citation2017). Annotation was in reference to A. bimaculata, L. decipiens (AB971912), and Eubasilissa regina (Trichoptera: Phryganeidae, NC023374) (Wang et al. 2014). The locations of mitochondrial tRNAs were determined using ARWEN v.1.2 (Laslett and Canback Citation2008). Also, the L. hyalinus nuclear rRNA repeat (GenBank MK077680) was assembled and annotated using A. bimaculata (MF680448) and Triaenodes tardus (Trichoptera: Leptoceridae, MG201853) (Lalonde and Marcus Citation2017) reference sequences.
The L. hyalinus circular 15,168 bp mitogenome assembly was composed of 10,042 paired reads with nucleotide composition: 39.5% A, 13.9% C, 8.1% G, and 38.4% T. Gene composition and order in L. hyalinus are identical to most other trichopteran mitogenomes (Marcus Citation2018). Limnephilus hyalinus COX1 begins with an aberrant start codon (CGA) like many other insects (Liao et al. Citation2010). The mitogenome contains three protein-coding genes (ATP8, NAD1, NAD5) with single-nucleotide (T) stop codons and one gene (NAD6) with a two-nucleotide (TA) stop codon completed by post-transcriptional addition of 3′ A residues. The structure and arrangement of tRNAs, rRNAs, and control region are typical for Trichoptera (Lalonde and Marcus Citation2017; Peirson and Marcus Citation2017). The mitogenome contains an mtTERM binding site (ATACTAATA) between tRNA-Ser and NAD1, similar to those described from sister Order Lepidoptera (McCullagh and Marcus Citation2015).
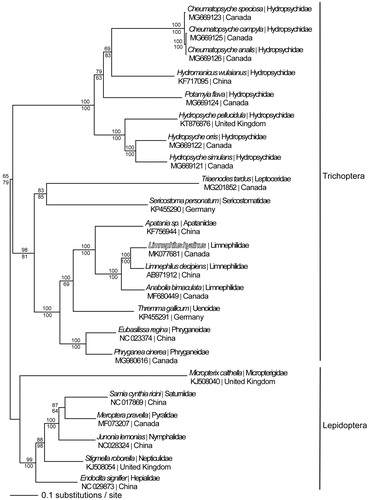
The mitogenomes from L. hyalinus, 16 other Trichoptera, and six Lepidoptera species were aligned in CLUSTAL Omega (Sievers et al. Citation2014) and analysed by maximum likelihood (ML) and parsimony in PAUP* 4.0b8/4.0d78 (Swofford Citation2002) (). Phylogenetic analysis reveals monophyletic Trichoptera and Limnephilidae and places L. hyalinus as sister to L. decipiens.
Figure 1. Maximum-likelihood phylogeny of superorder Amphiesmenoptera (GTR+I+G model, I = 0.1680, G = 0.8600, likelihood score 200095.94713) included complete mitochondrial genome sequences from Limnephilus hyalinus, 16 other Trichoptera species, and six representatives from sister clade Lepidoptera based on 1 million random addition heuristic search replicates (with tree bisection and reconnection). One million maximum parsimony heuristic search replicates also produced a single tree (43,649 steps) with a topology identical to the ML tree. Maximum-likelihood bootstrap values are above nodes and maximum parsimony bootstrap values are below nodes (each from 1 million random fast addition search replicates).
Acknowledgements
We thank Sarah Semmler and Kyle Lucyk for permitting and encouraging our work at the Living Prairie Museum. We thank Melissa Peters for help with fieldwork and Aleksandar Ilik and Debbie Tsuyuki (Children’s Hospital Research Institute of Manitoba Next Generation Sequencing Platform) for assistance with library preparation and sequencing.
Disclosure statement
The authors report no conflicts of interest and are solely responsible for this paper.
Additional information
Funding
References
- Houghton DC. 2012. Biological diversity of the Minnesota caddisflies (Insecta, Trichoptera). Zookeys. 189:1–389.
- Lalonde MLM, Marcus JM. 2017. The complete mitochondrial genome of the long-horned caddisfly Triaenodes tardus (Insecta: Trichoptera: Leptoceridae). Mitochondrial DNA B Resour. 2:765–767.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6:172–186.
- Living Prairie Mitogenomics Consortium. 2017. The complete mitochondrial genome of the lesser aspen webworm moth Meroptera pravella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2:344–346.
- Living Prairie Mitogenomics Consortium. 2018. The complete mitochondrial genome of the giant casemaker caddisfly Phryganea cinerea (Insecta: Trichoptera: Phryganeidae). Mitochondrial DNA B Resour. 3:375–377.
- Marcus JM. 2018. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 5:1–23.
- Marcus JM, Hughes TM, McElroy DM, Wyatt RE. 2010. Engaging first year undergraduates in hands-on research experiences: the Upper Green River Barcode of life project. J Coll Sci Teach. 39:39–45.
- McCullagh BS, Marcus JM. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae). J Asia-Pacific Ent. 18:749–755.
- McCullagh BS, Wissinger SA, Marcus JM. 2015. Identifying PCR primers to facilitate molecular phylogenetics in Caddisflies (order Trichoptera). Zool Syst. 40:459–469.
- Peirson DSJ, Marcus JM. 2017. The complete mitochondrial genome of the North American caddisfly Anabolia bimaculata (Insecta: Trichoptera: Limnephilidae). Mitochondrial DNA B Resour. 2:595–597.
- Peters MJ, Marcus JM. 2017. Taxonomy as a hypothesis: testing the status of the Bermuda buckeye butterfly Junonia coenia bergi (Lepidoptera: Nymphalidae). Syst Entomol. 42:288–300.
- Ruiter DE. 1995. The adult Limnephilus Leach (Trichoptera: Limnephilidae) of the New World. Bull Ohio Biol Surv N S. 11:1–200.
- Schwiebert E. 2007. Nymphs, stoneflies, caddisflies, and other important insects: including the lesser mayflies (volume II). Boston: Lyons Press.
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. 2014. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7:539.
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland (MA): Sinauer Associates.
- Wang Y, Liu X, Yang D. 2014. The first mitochondrial genome for caddisfly (Insecta: Trichoptera) with phylogenetic implications. Int J Biol Sci. 10:53–63.
- Winter WD. 2000. Basic techniques for observing and studying moths and butterflies, vol.5. Los Angeles (CA): The Lepidopterists' Society.
