Abstract
Siamese cobra (Naja kaouthia) exhibits highly toxic venom, which causes morbidity and mortality. Accurate species identification through molecular approaches is very important to administer correct antivenoms. The Siamese cobra mitogenome contains 17,203 bp with slight AT bias (58.2%) containing 37 genes in identical order to snake mitogenomes; no tandem repeat was found in the control region. Phylogenetic analysis indicated that Siamese and other cobras had highly supported monophyletic clades similar to the genus Naja and close relationships with other elapid snakes. Our results will facilitate clinical diagnosis and enrich genomic resources for future evolutionary studies and conservation management.
Siamese cobra (Naja kaouthia) is widely distributed across South and Southeast Asia. However, snake biodiversity is decreasing globally due to urbanization and hunting related to trade in health foods and medicinal products, resulting in severe decline of local populations (Liu et al. Citation2014). Siamese cobra exhibits highly toxic venom with its bite a serious daily occurrence, often causing morbidity and mortality even though specific anti-venom is available (Modahl et al. Citation2016). Accurate snake identification before application of anti-venom therapy is essential. Currently, clinical signs and molecular methods are not sufficient indicators (Supikamolseni et al. Citation2015). Genetic resource studies including molecular markers will provide important information for both conservation management and clinical species diagnosis. Here, we determined the complete mitochondrial genome (mitogenome) of Siamese cobra collected from a female individual (NK711) at Snake Farm, Queen Saovabha Memorial Institute and analyzed phylogenetic relationships among elapid species using available data in GenBank. Whole genomic DNA was extracted from blood samples in accordance with the standard salting-out protocol (Supikamolseni et al. Citation2015). Next-generation sequencing was performed using an Illumina HiSeq X Ten platform at Macrogen Sequencing Service (Macrogen, Geumcheon-gu, Seoul, Korea). Mapping was performed against the complete mitogenome of N. naja (GenBank accession number: DQ343648). High-quality reads were assembled using Velvet (Velvet_1.1.07; kmer = 91) (Zerbino and Birney Citation2008). 1,100 individual mitochondrial reads gave an average coverage of 100×, with annotations generated in MITOS WebServer (Bernt et al. Citation2013).
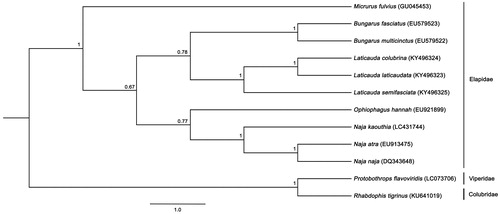
Complete mitogenome sequences consisted of 17,203 bp with AT-bias of 58.2% (GenBank accession number: LC431744). Mitogenomes contained 37 genes and two putative control regions (CRs). Gene arrangement patterns were identical to those of snakes (Yan et al. Citation2008; Qian et al. Citation2018). The ND6 gene and eight tRNA genes were encoded by the L-strand, with the remaining genes encoded by the H-strand. The two putative CRs (1,028 bp and 1,027 bp in length) were located between tRNA-Ile and tRNA-Leu for CR1 and between tRNA-Pro and tRNA-Phe for CR2, respectively, with three conserved sequence blocks: CSB-1, CSB-2, and CSB-3 in the two CRs of snake mitogenomes (Yan et al. Citation2008; Qian et al. Citation2018). No tandem repeat was found in either of the two CRs, a result similar to common snake mitogenomes (Qian et al. Citation2018). Comparison of overall nucleotide diversity among elapid snake mitogenomes was determined at 18.15 ± 0.66% and among Naja at 3.88%, suggesting sequence divergence in the same genus. A phylogenetic tree of 10 elapid snakes was constructed based on 12 concatenated protein-coding genes without ND6 for non-elapid snakes, with Rhabdophis tigrinus and Protobothrops flavoviridis as the outgroup using Bayesian inference, MrBayes version 3.2.6 (Huelsenbeck and Ronquist Citation2001). All cobras formed a monophyletic group as genus Naja, while other elapid snakes were positioned with cobras in the same major clade (). This mitogenome sequencing project is important to provide a ‘backbone’ for the genus by offering a major framework contribution for further understanding of snake diversification, conservation management, and diagnostic clinical applications.
Figure 1. Phylogenetic relationships among 12 concatenated mitochondrial protein-coding genes without ND6 sequences of 12 mitochondrial genomes including Rhabdophis tigrinus and Protobothrops flavoviridis as the outgroup, inferred using Bayesian inference analysis. The complete mitochondrial genome sequence was downloaded from GenBank. Accession numbers are indicated in parentheses after the scientific names of each species. Support values at each node are Bayesian posterior probabilities while branch lengths represent the number of nucleotide substitutions per site.
Ethical approval
Animal care and all experimental procedures were approved by the Animal Experiment Committee, Kasetsart University, Thailand (approval no. ACKU59-SCI-034 and no. ACKU61-SCI-024), and conducted in accordance with the Regulations on Animal Experiments at Kasetsart University.
Acknowledgements
We would like to thank Taksa Vasaruchapong and Panithi Laoungbua (Snake Farm, Queen Saovabha Memorial Institute, Thai Red Cross Society, Thailand) for assistance during sample collection.
Disclosure statement
The authors report no conflicts of interest and assume responsibility for the contents of this article.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755.
- Liu G, Yang Z, Chen B, Ulgiati S. 2014. Energy-based dynamic mechanisms of urban development, resource consumption and environmental impacts. Ecol Model. 271:90–102.
- Modahl CM, Mukherjee AK, Mackessy SP. 2016. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia). Toxicon. 119:8–20.
- Qian L, Wang H, Yan J, Pan T, Jiang S, Rao D, Zhang B. 2018. Multiple independent structural dynamic events in the evolution of snake mitochondrial genomes. BMC Genomics. 19:354.
- Supikamolseni A, Ngaoburanawit N, Sumontha M, Chanhome L, Suntrarachun S, Peyachoknagul S, Srikulnath K. 2015. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet Mol Res. 14:13981–13997.
- Yan J, Li H, Zhou K. 2008. Evolution of the mitochondrial genome in snakes: gene rearrangements and phylogenetic relationships. BMC Genomics. 9:569.
- Zerbino D, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
