Abstract
The poachers adopted various cunning ways to modify the wildlife materials before commercialization. Thus, the confiscated wildlife materials is often a crime with no eyewitness and difficult for identification. However, accurate identification of the seized wildlife samples is vital for species identification, detection of trade origin and better conservation management. The study employed DNA sequencing based identification of suspected wildlife materials, received from forest departments in east India. We adopted sufficient safety measures while dealing with the samples and processed the entire job at the in-house facilities in Zoological Survey of India (ZSI), Kolkata. Among 21 confiscated wildlife materials, most of them are identified by Cytb gene, while one specimen by COI gene, and one by both COI and Cytb. All the confiscated amorphous samples are accurately detected by sequence similarity search (99%–100%), estimated genetic divergence, and return monophyly using NJ phylogenetic analysis. The study detected the on-going illegal trade of five of east India’s most threatened animals, B. gaurus, N. nebulosa, R. unicornis, L. olivacea, and P. molurus. The study further identified the illegal hunting of mammals (S. scrofa, B. indicus, M. muntjak, A. axis), avifauna (G. leucolophus), and herpetofauna (N. naja) in the region. The study affirms the efficacy of DNA based identification tools for detection of wildlife crime and forensic sciences.
1. Introduction
Despite various stringent laws in operation, wild animals are declining due to overexploitation in both local and international trade that has drawn serious global attention (Alacs et al. Citation2010; Linacre and Tobe Citation2011). The global living planet index shows a decline of 52% in faunal species between 1970 and 2010 (WWF Citation2014). Nevertheless, many keystone species across the biomes are threatened by wildlife trade (TRAFFIC Citation2014). Among the extant animals in India, tiger, turtles, deer, rhinos, elephants, different cats, and porcupines are just a few of many examples of taxa; that are threatened globally due to enormous poaching. Eventually, various national and international regulations and treaties came into force leading to ratification and enactment of wildlife laws nationwide (CITES Citation2013; IUCN Citation2018). Like in other nations, many biological species are being protected in India under the ‘Wildlife Protection Act, 1972 (as amended up to date)’ (Negi Citation2013; Mishra et al. Citation2008). Despite the existence of strict legislation and imposition of penalties, the perpetrators are often acquitted due to lack of evidential support from accusers with regard to species identification. Thus, identification of animals, whether scheduled or not, is of pivotal importance in the court of law (Gupta Citation2012; Verma and Goswami Citation2014). The wildlife materials from the hunted animals are commercialized in different amorphous forms; like chopped meat, tanned skin, bones and horns, ivory products and so on (Kumar et al. Citation2014; Nishant et al. Citation2017). Due to the lack of morphological characters identification becomes inconclusive. Therefore, in order to recognize the source animal of traces seized from poachers’ hand, the conservation strategies warrant an accurate and rapid species-level identification system.
With the advent of DNA technology, several wildlife forensic cases have been expedited and settled worldwide and India as well (Ogden et al. Citation2009; Cooper et al. Citation2009). Plethora of instances using different molecular techniques are available for species-level identification (Hakki et al. Citation2003; Asmelash et al. Citation2017). In the recent past, the use of molecular markers (Cytochrome b, 12S ribosomal RNA, 16S ribosomal RNA, and Cytochrome oxidase c subunit I) also estimated as a successful tool for species identification due to the bearing of nucleotide variation among the species (Hsieh et al. Citation2001; Panday et al. Citation2014). Nevertheless, millions of gene sequences are presently available in global database (http://www.boldsystems.org, https://www.ncbi.nlm.nih.gov) and the online gene sequence similarity search engines are proved to be effective to examine the DNA data, generated from any biological sources. In this present study, we examined the wildlife seized materials, received from the different forest department of West Bengal state in east India, through DNA sequences. The molecular investigation was helped to identify the confiscated samples up to the species level. Some of the case studies of wildlife crime investigation; which essentially sought molecular intervention for species identification are presented herein. The present study evident the utility of DNA based investigation to identify the amorphous confiscated materials as well as in wildlife forensic sciences.
2. Materials and methods
2.1. Collection of confiscated wildlife sample
The confiscated wildlife materials were received from the different forests departments, and Wildlife Crime Control Bureau, West Bengal, Govt. of India. Most of the seized samples were received either in chopped form or partially amorphous, preserving in either alcohol or in low concentration formalin. Few wildlife materials (ZSI_MAM-11, ZSI_MAM-13, ZSI_MAM-14, and ZSI_MAM-22A) were cooked, confiscated from the rural villages in northern part of West Bengal. Two snake venom samples (ZSI_REP-16 and ZSI_REP-17) were received in semi-liquid condition. Two samples (ZSI_MAM-21C and ZSI_MAM-23) were fixed as dried skin with the hunting arrow, and one live specimen (ZSI_REP-19) was seized from the Kolkata snake park. The confiscated samples were received at Centre for DNA Taxonomy (CDT) laboratory, Zoological Survey of India (ZSI), Kolkata as completely sealed and returned to the concerned sender after properly storing in preservative. The subsamples were collected from each of the confiscated samples (raw meat, cooked meat, dried skin) for DNA study and immediately washed 3–4 times by 70% molecular grade ethanol and nuclease-free water (Gaur and Reddy 2017). While subsampling the received tissue samples, tried to remove the surface layer by using a sterile surgical blade in a separate room to avoid the contamination. For formalin stored samples, the tissue was vigorously washed by different concentration of alcohol to remove the formalin from tissue sample and then vortexed. Finally, the tissue samples were stored in 70% alcohol at −30 °C for molecular analysis. The cloacal swab sample from the live snake (ZSI_REP-19) was directly stored in commercialized tissue lysis ATL buffer and the venom samples are directly processed for DNA isolation. All tissue samples were stored in −80 °C at CDT, ZSI, Kolkata.
2.2. DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted in the separate DNA isolation room and the QIAamp DNA Investigator Kit as per the standard protocol and stored at Centre for DNA Taxonomy, ZSI, Kolkata. The genomic DNA was checked by 1% Agarose gel and the quantification of the extracted DNA was determined by using the Nanodrop (Eppendorf). According to the availability, two sets of primer pairs were used to amplify the partial mtCOI segment (Kocher et al. Citation1989; Nagy et al. Citation2012), one primer pair was used to amplify the partial mtCytb segment (Verma and Singh Citation2002) of mitochondrial gene in a Veriti® Thermal Cycler (Applied Bio systems, Foster City, CA). The 25 µl PCR mixture contains 10 pmol of each primer, 20 ng of DNA template, 1× PCR buffer, 1.0–1.5 mM of MgCl2, 0.25 mM of each dNTPs, and 0.25 U of Platinum Taq DNA Polymerase High fidelity (Invitrogen, Life Science Technologies). The PCR products were checked in 1% agarose gel containing ethidium bromide (10 mg/ml). Further, the PCR products were purified using QIAquickR Gel extraction kit (QIAGEN Inc., Germantown MD), and cycle sequencing products were cleaned by using standard BigDye X Terminator Purification Kit (Applied Biosystems, Foster City, CA). Sequencing was done bi-directionally in 48 capillary array 3730 DNA Analyzer (Applied Biosystems, Foster City, CA) following Sanger sequencing methods in the in-house sequencing facilities in the ZSI, Kolkata (Kundu et al. Citation2016).
2.3. Sequence quality control measures
The generated sequences were checked by Sequence Analysis software (ABI) and assured by the online BLAST search program (https://blast.ncbi.nlm.nih.gov) and ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Finally, the generated sequences were submitted in the global database (GenBank) to acquire the specific accession number. The species identification of the confiscated wildlife materials were preliminarily executed through online identification system, in GenBank with ‘Highly similar sequences (megablast)’. Further, we screened the GenBank database to acquire the publicly available COI and Cytb sequences of same and related species. The screened sequences were aligned using ClustalX software (Thompson et al. Citation1997) to make dataset specific to each taxon and molecular markers (COI and Cytb). The generated sequences were analyzed through neighbour-joining (NJ) tree with Kimura 2 parameter (K2P) by using MEGA6 to infer the genetic distance and monophyletic clustering of the studied taxa for accurate species-level identification (Tamura et al. Citation2013).
3. Results and discussion
The seized samples were received in the CDT laboratory, ZSI, Kolkata and the subsample of each was given a sample IDs that are presented in . The table also depicts the DNA source, sender details of confiscated materials in West Bengal state of east India, targeted gene for identifying the samples, accession numbers, and their conservation status in The IUCN Red List of Threatened Species, Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and Indian Wildlife Protection Act, 1972 (IWPA). The details of the DNA based investigation of the studied samples are discussed below.
Table 1. Details of the confiscated wildlife materials received at ZSI, Kolkata for species identification.
The generated Cytb sequence of ZSI_MAM-21C (MH117914) shows 99% similarity with the database sequence of Muntiacus muntjak (AF042715). The M. muntjak shows 1–2% genetic divergence within species and 7% to 9% with other Muntiacus congeners. The NJ phylogeny by Cytb gene also shows cohesive clustering of the studied sample with the database sequences and confirmed the species identity as M. muntjak, Indian Muntjac ().
Figure 1. The neighbour-joining trees of the studied confiscated wildlife materials for clustering based species identification. (A) Muntiacus muntjak (B) Bos gaurus (C) Lepidochelys olivacea (D) Python molurus (E) Sus scrofa (F) Naja naja (G) Garrulax leucolophus (H) Rhinoceros unicornis (I) Axis axis (J) Neofelis nebulosa. The generated sequences were marked by black dots in the trees and depicts the cohesive clustering with the available reference sequences acquired from GenBank database. The trees also represent the relationship of the examined species with other congeners.
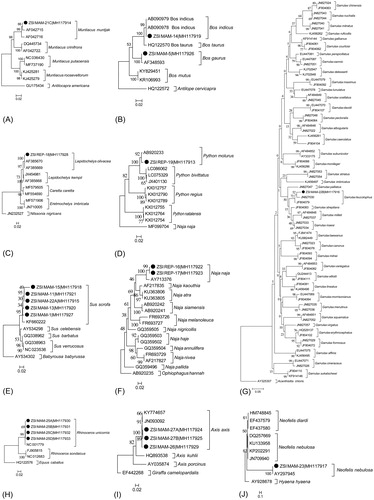
The generated COI sequence of ZSI_MAM-5 (KF808255) shows 99% to 100% similarity with Bos gaurus, B. indicus and B. frontalis. The COI sequence was not further incorporated in genetic divergence estimation and phylogenetic analysis. Further, the generated Cytb sequence of the sample (MH117926) shows 99% similarity with the database sequence of B. gaurus (AF348593). The generated Cytb sequence of ZSI_MAM-14 (MH117919) shows 99% similarity with the database sequence of B. indicus (AB090978). Both the generated sequences of Bos species shows identical genetic divergence (0%) and cohesive clustering with the representative database sequences in Cytb gene (). The two clades of B. gaurus, and B. indicus resulted in 7.3% genetic divergence in the studied dataset. Bos indicus is not yet assessed by any conservation agencies, however, the B. gaurus is categorized as threatened species globally as well as in India. Thus the on-going wildlife crime of B. gaurus, the Indian gaur should be monitored for better conservation management in India and other countries.
Further, the generated Cytb sequences of ZSI_REP-18 (MH117928) shows 100% similarity with the database sequence (AF385670) of Lepidochelys olivacea. The generated sequences show identical genetic divergence (0%) with the database sequence of L. olivacea and show cohesive clustering in the NJ tree by using Cytb gene (). The generated and database sequence further shows 1.9% genetic distance with the other congener L. kempii. Therefore, the confiscated samples were confirmed as the Olive Ridley sea turtle, L. olivacea.
The generated COI sequence of ZSI_REP-19 (MH117913) shows 99% similarity with the database sequence of Python molurus (AB920233). The generated sequence also shows 1.3% genetic divergence and cohesive clustering with the representative database sequences in the NJ tree by COI gene (). The Burmese python, P. bivittatus often confused with the P. molurus due to their similarities in morphological characters and overlap range distribution. The specimen is identified as the P. molurus, Indian rock python through molecular data with the sufficient genetic divergence (4.7%) with the P. bivittatus. Thus the on-going illegal trade of this highly threatened species must be monitored for better conservation management.
Further, the generated Cytb sequences of four samples (ZSI_MAM-1, ZSI_MAM-11, ZSI_MAM-13, and ZSI_MAM-15) 99% similarity search with the GenBank sequence of Sus scrofa (KT965278). Further, the generated sequences were resulted in 0% to 2.1% K2P genetic divergence and cohesive clustering with the database sequences of S. scrofa by using Cytb gene (). Based on the DNA data, we confirmed the seized samples as S. scrofa, Indian boar. The Indian S. scrofa further divided into two subspecies, S. s. davidi and S. s. cristatus. Although, the molecular data identify the seized materials up to the species level, but unable to confirm as wild boar. The wild boar is categorized in the Indian Wildlife Protection Act, 1972 as Schedule III species. However, due to the lack of subspecies level identification, the study could not confirm the accusation.
The two generated Cytb sequences of ZSI_REP-16 (MH117922), and ZSI_REP-17 (MH117923) shows 100% similarity with the database sequence (AY713376) of Naja naja. The generated sequences show 3.5% genetic divergence with the database sequence of N. naja and show cohesive clustering in the NJ tree by Cytb gene (). Therefore, the confiscated samples were confirmed as the Indian spectacled cobra, N. naja. The species often confused with N. atra and N. kaouthia, while missing the morphological characters, or road-killed samples. Thus, the generated sequence of N. naja will further help in species identification.
Further, the generated Cytb sequence of ZSI_MAM-22B (MH117916) shows 96% similarity with the database sequence of Garrulax leucolophus (JN827030). Due to the low similarity search in the GenBank database, the study acquired available representative sequences of all Garrulax species in the present dataset. The generated sequence show high genetic divergence (2.2–3.4%) with the two database sequences of G. leucolophus and 5.7–32.2% with other Garrulax species. The NJ tree by Cytb gene also depicts cohesive clustering of the studied sample with G. leucolophus and confirmed as the same species, White-crested laughingthrush ().
The four generated Cytb sequences of ZSI_MAM-25A (MH117930), ZSI_MAM-25B (MH117931), ZSI_MAM-25C (MH117932), and ZSI_MAM-25D (MH117933) shows 99% to 100% similarity of the database sequence of Rhinoceros unicornis (NC_001779). The generated sequences also show 0–0.3% genetic distance with the database sequence of R. unicornis and 9.3% to 9.6% with the Javan Rhinoceros, R. sondaicus. The NJ tree by Cytb gene also shows cohesive clustering of the generated sequences and database sequence with 99 bootstrap support (). The Greater One-horned Indian Rhinoceros, R. unicornis is highly threatened and native to India with narrow distribution. However, the smuggling of the species is an alarming issue in Southeast Asian countries including India. The horn of the species is highly demanding in black market and erroneously believe for medicinal value. The generated sequences in the present study will help to identify the threatened species and monitoring for better conservation.
Further, the three generated Cytb sequences of ZSI_MAM-26 (MH117929), ZSI_MAM-27A (MH117924), and ZSI_MAM-27B (MH117925) shows 99% similarity with the database sequence (KY774657) of Axis axis. The generated sequences show 0.5% genetic divergence with the database sequence of A. axis and show cohesive clustering in the NJ tree by Cytb gene (). The dataset shows 8.6% to 14.2% genetic divergence between the congeners of Axis. Therefore, the confiscated samples were confirmed as the Indian Chital, A. axis.
The generated Cytb sequence of ZSI_MAM-23 (MH117917) shows 100% similarity with the database sequence of Neofelis nebulosa (DQ257669 and AY297945). The generated sequence of the studied sample resulted in 1.33% genetic divergence with the database sequence (DQ257669, generated from China) and identical genetic divergence (0%) with the sequence (AY297945, generated from India). Further, the generated and database sequences show two distinct clades of N. nebulosa in the present dataset by using Cytb gene with low bootstrap support (). However, more DNA data of the species from a wide geographical area might help to reconcile the phylogeny. The species is highly threatened with long distribution range in South and Southeast Asian countries. This generated sequence of this species might be helpful for future species identification and monitoring the illegal trade and poaching of the species.
The aimed study accurately identified all confiscated wildlife samples up to the species level. This is the first initiative of ZSI as the identification of wildlife samples through DNA based investigation and received attention as one of the forensic laboratories in the country. The contemporary research and development facilities in ZSI with experienced experts in both taxonomy and molecular-based species identification research; further helps to examine many wildlife forensic cases and assists many organizations for detection of illegal wildlife crime and trade.
Acknowledgements
We thank the Director of Zoological Survey of India, Kolkata, Ministry of Environment, Forest and Climate Change, New Delhi for providing necessary permissions and facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alacs EA, Georges A, FitzSimmons NN, Robertson J. 2010. DNA detective: a review of molecular approaches to wildlife forensics. Forensic Sci Med Pathol. 6:180.
- Asmelash B, Diriba S, Pal SK. 2017. Molecular markers based characterization and conservation of wild animals. Res J Recent Sci. 6:53–62.
- CITES. 2013. Amendments to Appendices I and II of the Convention adopted by the Conference of the Parties at its 16th meeting, Bangkok, Thailand, 3–14 March 2013. Notification to the Parties No. 2013/20. Geneva, Switzerland.
- Cooper JE, Cooper ME, Budgen P. 2009. Wildlife crime scene investigation: techniques, tools and technology. Endang Species Res. 9:229–238.
- Gaur A, Reddy A. 2017. DNA Techniques in Wildlife Forensics (Animals): Standard Operating Procedures (SOP). CSIR Centre for Cellular and Molecular Biology, Hyderabad. 37 p.
- Gupta SK. 2012. DNA wildlife forensics: present and future. J Forensic Res. 3:e103. doi:10.4172/2157-7145.1000e103.
- Hakki EE, Uz E, Sag A, Atasoy S, Akkaya MS. 2003. DNA fingerprinting of Cannabis sativa L. accessions using RAPD and AFLP markers. Forensic Sci Int. 136:31.
- Hsieh HM, Chiang HL, Tsai LC, Lai SY, Huang NE, Linacre A, Lee JC. 2001. Cytochrome b gene for species identification of the conservation animals. Forensic Sci Int. 122:7–18.
- IUCN. 2018. The IUCN red list of threatened species, Version 2017.3. [accessed 2018 July 8]. https://www.iucnredlist.org
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 86:6196–6200.
- Kumar VP, Kumar D, Goyal SP. 2014. Wildlife DNA forensic in curbing illegal wildlife trade: species identification from seizures. Int J Forensic Sci Pathol. 2:38–42.
- Kundu S, Kumar V, Laskar BA, Chandra K, Tyagi K. 2016. Mitochondrial DNA effectively detects non-native testudines: invisible wildlife trade in northeast India. Gene Rep. 4:10–15.
- Linacre A, Tobe SS. 2011. An overview to the investigative approach to species testing in wildlife forensic science. Investig Genet. 2:2.
- Mishra P, Subramanian A, Topalova P. 2008. Tariffs, enforcement, and customs evasion: evidence from India. J Public Econ. 92:1907–1925.
- Nagy ZT, Sonet G, Glaw F, Vences M. 2012. First large-scale DNA barcoding assessment of reptiles in the biodiversity hotspot of madagascar, based on newly designed COI primers. PLoS ONE. 7:e34506.
- Negi SB. 2013. Wildlife crime investigation a hand book for wildlife crime investigation officers. New Delhi: Wildlife Crime Control Bureau, Ministry of Environment and Forests Government of India.
- Nishant K, Vrijesh KY, Ajay KR. 2017. Wildlife forensic: current techniques and their limitations. J Forensic Sci Criminol. 5:402.
- Ogden R, Dawnay N, McEwing R. 2009. Wildlife DNA forensics-bridging the gap between conservation genetics and law enforcement. Endang Species Res. 9:179–195.
- Panday R, Jha DK, Thapa N, Pokharel BR, Aryal NK. 2014. Forensic wildlife parts and their product identification and individualization using DNA barcoding. The Open Forensic Sci J. 7:6–13.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882.
- TRAFFIC. 2014. Traffic international. [accessed 2018 July 8]. [www.traffic.org.]
- Verma SK, Goswami GK. 2014. DNA evidence: current perspective and future challenges in India. Forensic Sci Int. 241:183–189.
- Verma SK, Singh L. 2002. Novel universal primers establish identity of an enormous number of animal species for forensic application. Mol Ecol Notes. 3:28–31.
- WWF. 2014. Living Planet Report 2014: Summary. McLellan R, Iyengar L, Jeffries B, Oerlemans N, editors. Gland, Switzerland: WWF.
