Abstract
Calocedrus rupestris is listed as endangered on the IUCN Red List. In this study, we report the complete chloroplast genome (plastome) of C. rupestris. The plastome was 127,298 bp in length and encodes 118 unique genes, including 83 protein-coding genes (PCGs), 31 tRNAs, and 4 rRNAs. Nine PCGs contained one intron each and two PCGs contained two introns each. Six tRNAs contained one intron each. The overall GC content of this plastome was 34.8%. The phylogenomic analysis revealed that C. rupestris was sister to C. macrolepis and C. formosana with strong support.
Calocedrus rupestris is a conifer species distributed in limestone mountains in northern Vietnam (Averyanov et al. Citation2005) and China (Guangxi and Guizhou) (Nong et al. Citation2011; Hoch Citation2017). It was first described in 2004. C. rupestris was classified as endangered on the IUCN Red List of Threatened Species in 2013 (Thomas et al. Citation2013). In this study, we reported the plastome of C. rupestris, which will contribute to propose protection strategies for this endangered species.
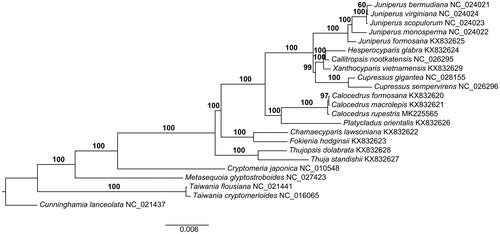
Fresh leaves of Calocedrus rupestris were collected from Libo County (Guizhou, China; 25°12′26″N, 107°55′16″E). Voucher specimen (Yi13630) was deposited at Kunming Institute of Botany Chinese Academy of Sciences. Total genomic DNA was isolated using Plant Genomic DNA Kits (TIANGEN, Beijing, China) described in Zhao et al. (Citation2017). We do not directly extract the plastid DNA (Liu et al. Citation2017) due to limited fresh material. Then the total genomic DNA was used for library preparation and paired-end sequencing by the Illumina MiSeq instrument at Kunming Institute of Botany (Kunming, China). Then, the plastome was assembled using the Organelle Genome Assembler (OGA) pipeline (https://github.com/quxiaojian/OGA). First, Bowtie v2.3.4 (Langmead and Salzberg Citation2012) was used for mapping raw reads against the reference plastome of C. macrolepis (Qu, Wu, et al. Citation2017). Second, Spades v3.7.1 (Bankevich et al. Citation2012) was used to assemble mapped reads into contigs. Third, the Perl script OGA.pl was used to recruit plastome-like reads based on the overlap between contigs and raw reads. Fourth, the mapped and recruited reads were assembled into final contigs using Spades. Finally, the path of plastome contigs was connected by Bandage v8.0 (Wick et al. Citation2015). Annotation was performed with a newly developed software package Plastid Genome Annotator (PGA, https://github.com/quxiaojian/PGA), coupled with manual correction using Geneious v9.1.4 (Kearse et al. Citation2012). Physical map of the plastome was drawn with OGDRAW (Lohse et al. Citation2013). To determine the phylogenetic placement of C. rupestris, a maximum likelihood (ML) tree was reconstructed using RAxML v8.2.10 (Stamatakis Citation2014), including tree robustness assessment using 1000 rapid bootstrap replicates with the GTRGAMMA substitution model, based on alignment of 73 shared protein-coding genes (PCGs) (Qu, Jin, et al. Citation2017) using MAFFT (Katoh and Standley Citation2013).
The complete plastome of C. rupestris (GenBank accession number: MK225565) was 127,298 bp in length and the overall GC content was 34.8%. C. rupestris, like other cupressophytes, lack one copy of the common inverted repeat regions. A total of 118 unique genes were annotated in this plastome, including 83 PCGs, 31 tRNAs, and 4 rRNAs. Among them, 11 PCGs contained introns, in which 9 PCGs (atpF, ndhA, ndhB, petB, petD, rpl16, rpl2, rpoC1 and rps16) contained 1 intron and 2 PCGs (rps12 and ycf3) contained 2 introns. The ML phylogenetic tree showed that C. rupestris was sister to C. macrolepis and C. formosana with strong support (). This complete plastome will be useful for future population genomic studies, and will provide essential data for the conservation and utilization of this endangered species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Averyanov LV, Nguyen TH, Phan KL, Pham VT. 2005. Distribution, habitat and ecology of Calocedrus rupestris (Cupressaceae) in Vietnam. Turczaninowia. 8:19–35.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Hoch J. 2017. Extended range area of Calocedrus rupestris in China. Bull CCP. 6:51–61.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649.
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359.
- Liu F, Jin Z, Wang Y, Bi YP, Melton JT. 2017. Plastid genome of Dictyopteris divaricata (Dictyotales, Phaeophyceae): understanding the evolution of plastid genomes in brown algae. Mar Biotechnol. 19:627–637.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. OrganellarGenomeDRAW-a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:W575–W581.
- Nong DX, Wu WH, Jiang RH, Huang YS, Xu WB. 2011. Notes on Calocedrus (Cupressaceae) in Guangxi, China. Guihaia. 31:155–159.
- Qu XJ, Jin JJ, Chaw SM, Li DZ, Yi TS. 2017. Multiple measures could alleviate long-branch attraction in phylogenomic reconstruction of Cupressoideae (Cupressaceae). Sci Rep. 7:41005.
- Qu XJ, Wu CS, Chaw SM, Yi TS. 2017. Insights into the existence of isomeric plastomes in Cupressoideae (Cupressaceae). Genome Biol Evol. 9:1110–1119.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Thomas P, Nguyen TH, Phan KL, Nguyen QH. 2013. Calocedrus rupestris. The IUCN Red List of Threatened Species 2013. eT133722A512509. http://dxdoiorg/102305/IUCNUK2013-1RLTST133722A512509en.
- Wick RR, Schultz MB, Zobel J, Holt KE. 2015. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 31:3350–3352.
- Zhao CZ, Qiu JJ, Agarwal G, Wang JS, Ren XZ, Xia H, Guo BZ, Ma CL, Wan SB, Bertioli DJ, et al. 2017. Genome-wide discovery of microsatellite markers from diploid progenitor species, Arachis duranensis and A. ipaensis, and their application in cultivated peanut (A. hypogaea). Front Plant Sci. 8:1209.