Abstract
The greater hog badger, Arctonyx collaris is a venerable and widely distributed terrestrial carnivore mammal in Indo-Burma biodiversity hotspot. The population of A. collaris has largely declined in its range distribution due to the habitat loss and bushmeat crisis. The present study identified the amorphous burnt body parts of a mammal as A. collaris, through mitochondrial cytochrome b (Cytb) gene sequences. The mustelids carnivores (family Mustelidae) with 51 species showed 0–3.03% intraspecies genetic distance in the present dataset. Further, the generated sequences of A. collaris from Manipur state showed notable genetic distance (2 and 3.5%) in comparison with Thailand and Vietnam specimens, which assumed to be a distinct meta-population in northeast India. However, additional genetic information of this carnivore mammal from the wide geographical area might be helpful for estimating their population structure and conservation genetics. The similar approach with different molecular markers would be useful in wildlife forensics by identifying the species, track the trade route, and also find the native population of many abused animals.
Introduction
Illegal hunting of wildlife animals is enormously declining the extant terrestrial mammal population throughout the world. The recent study estimated that 301 mammal species are threatened with extinction due to the illegal hunting for food, medicinal products, ornamental use, and the pet trade (Ripple et al. Citation2016). The species belonging to class primates, ungulates, marsupials, rodents, and carnivores are largely hunted mammals in Southeast Asian and African countries (TRAFFIC 2014). The keystone species have mostly accredited impacts on their exploitation and conservation activities due to indicators of biological process on its eco-regions (WWF Citation2014). Nevertheless, the less charismatic species are ignored every time to recognize their misuses, like wildlife trading and other unlawful activities (Fa et al. Citation2002). In India, the wildlife derived products such as skin (tiger, leopard, crocodile, python, monitor lizard), hair (Tibetan antelope, mongoose), meat (rodents, deers, turtles and tortoises, wild boar), pet (birds, primates, reptiles, scorpions), bear gall bladder, elephant ivory, rhinoceros horn, pangolin scale, and deer musk are repeatedly used for bushmeat, traditional medicines, and luxury purposes (Negi Citation2013). Despite the existing Wildlife Protection Act 1972, regulations and enforcement of international conservation legislation, wildlife trafficking still continues in different regions of India (CITES Citation2017).
In Southeast Asia, the Indo-Burma biodiversity hotspot is regarded as one of the prolific biodiversity rich zones and safe abode of many endemic and Threatened mammals including mustelids carnivores (Wozencraft Citation1993). This rich group is classified into 59 species under 22 genera of six subfamilies; Lutrinae (otters), Melinae (badgers), Mellivorinae (honey badger), Mephitinae (skunks), Mustelinae (weasels and martens), and Taxiidinae (American badger) (Koepfli et al. Citation2008). Among them, the hog badger, Arctonyx collaris is the only extant species in the monotypic genus Arctonyx. The hog badger is distributed in Bangladesh, India, Myanmar, Thailand, Laos, Vietnam, and Cambodia. Due to the decreasing population of A. collaris (assessed by IUCN/SSC Small Carnivore Specialist Group in 2015), the species was classified under ‘Vulnerable’ category in IUCN Red List of Threatened Species (IUCN Citation2018). It is also reported that these medium-sized carnivores have confronted several threats like habitat destruction due to urbanization, mining, construction of road, logging, and wood harvesting (Helgen et al. Citation2008). In fact, as the body parts of hog badger are not lucrative in the national/international market, hunting and trading are restricted locally (Chen et al. Citation2015).
The DNA barcode is evidenced as an effective tool for identification of large carnivores, lion and leopard, from body parts and wildlife forensics in India (Verma and Goswami Citation2014; Khedkar et al. Citation2016). Further, the mitochondrial cytochrome b (Cytb) gene is largely used to identify various mammals, including badgers (Koepfli and Wayne Citation1998; Hsieh et al. Citation2001). The use of universal primer pairs and amplifying the short fragment of the Cytb gene (∼450 bp) was successfully evidenced for wildlife identification (Verma and Singh Citation2002). Hence, the present study was aimed, (i) to identify the mammal species from an amorphous burnt body part through partial Cytb gene sequences and (ii) check the efficacy of short Cytb fragment to discriminate the identified species from other closely related mammals by estimating genetic distance and phylogeny.
2. Materials and methods
2.1. Sample collections
The amorphous leg part was collected from the nearby locality (25.13N 93.61E) of Bunning wildlife sanctuary in Tamenglong district of Manipur. No prior permission is acquired in this study as the wildlife samples were collected unexpectedly from the forest area during the field survey conducted by the Zoological Survey of India (ZSI). No hog badger specimens were sacrificed in the present study. However, based on the external structure (burning skin and sharp edge), we assumed that the animal was hunted and killed by human for bushmeat purposes. Due to the lack of hair in the sample, we adjudicated to adopt a molecular approach for identification rather than taxidermy. The sample was preserved in 70% molecular grade ethanol with proper voucher ID (ZSI/28309) in the National Zoological collections at Mammals and Osteology section, ZSI, Kolkata. Two subsamples (ZSI_MBT-1, and ZSI_MBT-2) with a meager amount of tissue were collected from the surface layer and the bone adjacent area by using sterile surgical blades for further molecular experiment.
2.2. DNA extraction, PCR amplification, and sequencing
After rigorous washing by 70% ethanol, total genomic DNA was extracted from the tissue samples by standard phenol-chloroform isoamyl alcohol (PCI) methods (Sambrook and Russell Citation2001) and checked in 1% Agarose gel. The published primer pair, mcb 398: 5′-TACCATGAGGACAAATATCATTCTG-3′ and mcb 869: 5′-CCTCCTAGTTTGTTAGGGATTGATCG-3′ was used to amplify the partial mitochondrial cytochrome b (Cytb) gene segment in a Veriti® Thermal Cycler (Applied Biosystems, Foster City, CA) with the standard thermal profile (Verma and Singh Citation2002). The 30 µL PCR mixture contains 10 pmol of each primer, 20 ng of DNA template, 1X PCR buffer, 1.0–1.5 mM of MgCl2, 0.25 mM of each dNTPs, and 1U of Taq polymerase (Takara BIO Inc., Shiga, Japan). The PCR products were checked in 1% agarose gel and purified using a QIAquickR Gel extraction kit (QIAGEN Inc., Germantown, MD). The amplicon is bidirectionally sequenced using Sanger Sequencing chemistry on 3730 DNA Analyzer (Applied Biosystems, Foster City, CA) platform available at ZSI, Kolkata.
2.3. Sequence quality control and dataset preparation
Both the generated forward and reverse chromatograms of each subsample were checked by using the software SeqScanner Version 1.0 (Applied Biosystems Inc., CA, USA). Further, the consensus sequences were assembled by BioEdit v7.2.5 (Hall Citation1999) and checked through the online nucleotide BLAST program (https://blast.ncbi.nlm.nih.gov/), and ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/) to examine the insertion-deletion and start-stop codons. The final sequences were submitted in the GenBank database to obtain the accession numbers. The generated sequences were primarily recognized through the online identification systems in global databases (GenBank and BOLD). A dataset of 79 sequences (including two sequences generated under this study) was prepared and sequences were aligned using ClustalX software (Thompson et al.Citation1997). Final sequence assembly was obtained having a uniform sequence length of 405 bp for further analysis. The Cytb sequence of Panthera tigris (AF053051) (family Felidae) was incorporated in the dataset as an outgroup. The genetic distance and topology were estimated through the Kimura 2 parameter (K2P) and neighbor-joining (NJ) tree by using MEGAX (Kumar et al. Citation2018).
3. Results and discussion
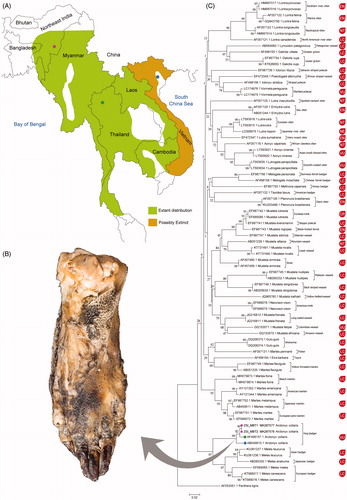
The generated sequences (ZSI_MBT-1: MK287577, and ZSI_MBT-2: MK287578) showed 98% similarity with the database sequence of Arctonyx collaris (AF498157). Hence, the present study accurately identified the burnt wildlife samples as the greater hog badger (A. collaris). The overall mean genetic distance of the dataset was 17.5%. Excluding the singleton sequences of 25 species from the dataset, the intraspecies genetic distance was ranging from 0 to 3.03%. The specimen of A. collaris (AF498157), collected from the Mai Zoo, Chiang, Thailand showed 2% genetic distance with the specimens collected from the Manipur state. However, the other specimen of A. collaris (AB049810), preserved in Museum of Vertebrate Zoology, University of California, Berkeley was collected from Vinh Yen district, Vietnam and maintained 3.5% genetic distance with the studied sample. The previous study suggested that the low-genetic divergence was detected in European badger, Meles canescens (0.2%) collected from two distant countries, Iran (KT988017) and Turkey (KT988016) (Ibiş et al. Citation2015). Hence, the high intraspecies genetic distance (3.03%) revealed within A. collaris sequences, suggested that the surveyed hog badgers might belong to different meta-population in Manipur state of northeast India, which needs further investigation by extensive sampling and use of more molecular markers.
Although the partial gene sequences of a single mitochondrial gene are useful for species identification, the concatenation of multiple genes substantiates better understanding of the phylogenetic relationship and other systematics queries (Kurose et al. Citation2001; Yu et al. Citation2004, Citation2011). The previous study with multiple genes demonstrated that the diversification of Mustelidae is an outstanding example of adaptive radiation and thus determining their phylogenetic relationships is challenging due to rapid cladogenesis (Schluter Citation2000). Further, both nuclear and mitochondrial Cytb genes were also used to resolve the phylogenetic relationships of mustelids carnivores (Koepfli and Wayne Citation2003). The present NJ tree with mitochondrial Cytb gene showed distinct clade of A. collaris by the generated and database sequences. The A. collaris depicted close relationship with other sister species, Meles leucurus, Meles anakuma, Meles meles, and M. canescens in the NJ phylogeny (). Further, all the 77 sequences of 51 Mustelids carnivores revealed discrete clustering in the phylogenetic analysis.
Figure 1. (A) Extant and possibly extinct range distribution of Arctonyx collaris in Southeast Asia (map not to scale) (B) Amorphous burnt body part of mammals collected from Manipur state in northeast India. (C) Neighbor-joining tree shows distinct clustering of A. collaris and other mustelid carnivores by mitochondrial cytochrome b gene. The database sequence of Panthera tigris (family Felidae) was used as an outgroup in the phylogeny. The color dots used in A. collaris clade node denoted the collection localities as marked in the map. The IUCN status of all the studied carnivores including A. collaris superimposes with each clade.
In recent decades, the integrated approach, combining both morphology and molecular techniques has become a dominant tool in biodiversity research. Both nuclear and mitochondrial gene sequences immensely proved its utility for assessing several biological questions like species identification (Hebert et al. Citation2003), uncovering the cryptic diversity (Tyagi et al. Citation2017), population genetics (Khalili Samani et al. Citation2016), and phylogenetic relationship (Laskar et al. Citation2018). Further, the assessment of the genetic diversity of any Threatened species is one of the principal parameters for conservation measurements. The contemporary molecular technologies have assisted in wildlife forensics by identifying the species and origin of the confiscated animals or products (Dawnay et al. Citation2008; Alacs et al. Citation2010). Further, the genetic information not only distinguished the trade route but also helped to find the native population, where the seized living animals can be released back (Gupta Citation2012). Henceforth, to estimate the comprehensive genetic diversity and population structure of A. collaris, more biological samples are required from different geographical regions. In conclusion, the present case study elucidates the illegal hunting of hog badger from the northeastern states in India. Therefore, to save these Threatened mammal group, it is necessary to conduct more awareness activities targeted to the local people and wildlife traffickers in the region. In addition, the study strongly encouraged the wildlife and forest departments for implementing a prompt action plan to ban wildlife trafficking in Manipur state and other parts of northeast India. This present approach can be applied to identify other confiscated wildlife animals and facilitate to develop precise conservation policies.
Acknowledgements
The authors are thankful to the Director, Zoological Survey of India (ZSI), Ministry of Environment, Forests and Climate Change (MoEF&CC), Govt. of India for providing necessary facilities, constant support, and encouragement throughout the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alacs EA, Georges A, FitzSimmons NN, Robertson J. 2010. DNA detective: a review of molecular approaches to wildlife forensics. Forensic Sci Med Pathol. 6:180.
- Chen W, Newman C, Liu Z, Kaneko Y, Omote K, Masuda R. 2015. The illegal exploitation of hog badgers (Arctonyx collaris) in China: genetic evidence exposes regional population impacts. Conservation Genet Resour. 7:697–704. doi:10.1007/s12686-015-0467-x.
- CITES. 2017. The CITES appendices; [2018 December 10]. http://cites.org.cn/database/index.php.
- Dawnay N, Ogden R, Thorpe RS, Pope LC, Dawson DA, McEwing R. 2008. A forensic STR profiling system for the Eurasian badger: a framework for developing profiling systems for wildlife species. Forensic Sci Int Genet. 2:47–53.
- Fa JE, Peres CA, Meeuwig J. 2002. Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv Biol. 16:232–237.
- Gupta SK. 2012. DNA wildlife forensics: present and future. J Forensic Res. 3:e103.
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 41:95–98.
- Hebert PD, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Philos Trans R Soc London B Biol Sci. 270:313–322.
- Helgen KM, Lim NTL, Helgen LE. 2008. The hog badger is not an edentate: systematics and evolution of the genus Arctonyx (Mammalia: Mustelidae). Zool J Linn Soc. 154:353–385.
- Hsieh HM, Chiang HL, Tsai LC, Lai SY, Huang NE, Linacre A, Lee JC. 2001. Cytochrome b gene for species identification of the conservation animals. Forensic Sci Int. 122:7–18.
- Ibiş O, Tez C, Özcan S, Yorulmaz T, Kaya A, Mohradi M. 2015. Insights into the Turkish and Iranian badgers (the genus Meles) based on the mitochondrial cytochrome b gene sequences. Vertebr Zool. 65:399–407.
- IUCN. 2018. The IUCN red list of Threatened species, Version 2017.3. Accessed on December 10, 2018.
- Khalili Samani N, Esa Y, Nurul Amin SM, Mohd Ikhsan NF. 2016. Phylogenetics and population genetics of Plotosus canius (Siluriformes: Plotosidae) from Malaysian coastal waters. PeerJ. 4:e1930. doi: 10.7717/peerj.1930.
- Khedkar GD, Abhayankar SB, Nalage D, Ahmed SN, Khedkar CD. 2016. DNA barcode based wildlife forensics for resolving the origin of claw samples using a novel primer cocktail. Mitochondrial DNA A DNA Mapp Seq Anal. 27:3932–3935.
- Koepfli K-P, Deere KA, Slater GJ, Begg C, Begg K, Grassman L, Lucherini M, Veron G, Wayne RK. 2008. Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biology. 6:10.
- Koepfli KP, Wayne RK. 2003. Type I STS markers are more informative than cytochrome b in phylogenetic reconstruction of the Mustelidae (Mammalia: Carnivora). Syst Biol. 52:571–593.
- Koepfli KP, Wayne RK. 1998. Phylogenetic relationships of otters (Carnivora: Mustelidae) based on mitochondrial cytochrome b sequences. J Zool. 246:401–416.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Kurose N, Kaneko Y, Abramov AV, Siriaroonrat B, Masuda R. 2001. Low genetic diversity in Japanese populations of the Eurasian badger Meles meles (Mustelidae, Carnivora) revealed by mitochondrial cytochrome b gene sequences. Zool Sci. 18:1145–1151.
- Laskar BA, Kumar V, Kundu S, Tyagi K, Chandra K. 2018. Taxonomic quest: validating two mahseer fishes (Actinopteri: Cyprinidae) through molecular and morphological data from biodiversity hotspots in India. Hydrobiologia. 815:113–124.
- Negi SB. 2013. Wildlife crime investigation: a hand book for wildlife crime investigation officers. New Delhi: Wildlife Crime Control Bureau, Ministry of Environment and Forests Government of India.
- Ripple WJ, Abernethy K, Betts MG, Chapron G, Dirzo R, Galetti M, Levi T, Lindsey PA, Macdonald DW, Machovina B, et al. 2016. Bushmeat hunting and extinction risk to the world's mammals. R Soc Open Sci. 3:160498.
- Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
- Schluter D. 2000. The ecology of adaptive radiation. Oxford: Oxford University Press.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882.
- TRAFFIC. 2014. Traffic international. https://doi.org/www.traffic.org. Accessed on December 10, 2018.
- Verma SK, Goswami GK. 2014. DNA evidence. Current perspective and future challenges in India. Forensic Sci Int. 241:183–189.
- Tyagi K, Kumar V, Singha D, Chandra K, Laskar BA, Kundu S, Chakraborty R, Chatterjee S. 2017. DNA barcoding studies on Thrips in India: cryptic species, species complexes. Sci Rep. 7:1–14.
- Verma SK, Singh L. 2002. Novel universal primers establish identity of an enormous number of animal species for forensic application. Mol Ecol Notes. 3:28–31.
- Wozencraft WC. 1993. Order Carnivora. In Wilson DE, Reeder DM, editors.Mammal Species of the World, Second Edition. Washington and London: Smithsonian Institution Press, p. 279–348.
- WWF. 2014. Living Planet Report 2014: species and spaces, people and places. In McLellan R, Iyengar L, Jeffries B, Oerlemans N, editors. Gland, Switzerland: WWF.
- Yu L, Li Q, Ryder OA, Zhang Y. 2004. Phylogenetic relationships within mammalian order Carnivora indicated by sequences of two nuclear DNA genes. Mol Phylogenet Evol. 33:694–705.
- Yu L, Luan P-T, Jin W, Ryder OA, Chemnick LG, Davis HA, Zhang Y-P. 2011. Phylogenetic utility of nuclear introns in interfamilial relationships of Caniformia (Order Carnivora). Syst Biol. 60:175–187.
