Abstract
The complete mitogenome of Triplophysa cuneicephala is 16,571 bp in length with the A + T contents of 56.12%, containing 13 protein-coding genes (PCGs), 22 tRNA genes, 2 rRNA genes, and a putative control region. All PCGs employed ATG as the start codon, except for COI which utilized GTG as start codon. Four stop codons were observed in the PCGs, including TAA, TAG, TA and T. Phylogenetic analysis based on concatenated PCG sequences revealed that T. cuneicephala is closely related to T. robusta and T. minxianensis. This study would provide useful genetic information for future studies on phylogeny, evolutionary, and taxonomic classification in Triplophysa species.
The genus Triplophysa is mainly distributed in the Qinghai-Tibet Plateau (QTP) and its adjacent regions with only a few species living in the regions at lower altitudes and far from QTP (Zhu Citation1989). Based on the biogeography studies, the origin and evolution of Triplophysa species are considered to be related to the uplift of the QTP (Zhu Citation1989; Wu and Wu Citation1992). During the last decade, mitochondrial genomes have been sequenced for many Triplophysa species (Li et al. Citation2013; Chen et al. Citation2016; Jing et al. Citation2016; Wang et al. Citation2016). However, among Triplophysa species that inhabit low altitudes, the information of mitogenome is only available for T. sellaefer (Feng et al. Citation2019), limiting our understanding of phylogenetic relationships of these species. In this study, we firstly sequenced and described the complete mitochondrial genome from T. cuneicephala which is mainly distributed in the Yongding River and its adjacent regions.
The specimens of T. cuneicephala used in this study were collected from the Juma River (N 39°25′32.31″, E 115°3′33.28″), Baoding city, China, and stored in 100% alcohol at −20 °C. The total genomic DNA was extracted from the muscle using a traditional phenol-chloroform extraction method (Taggart et al. Citation1992). The primer pairs used for amplifying the mitochondrial genome sequence were according to Feng et al. (Citation2019). The polymerase chain reaction (PCR) was performed according to our previous method (Feng et al. Citation2018). The remaining genomic DNA is currently stored at Institute of Hydrobiology, Chinese Academy of Sciences. After DNA sequencing, the complete mitochondrial genome was assembled and annotated.
The complete mitogenome of T. cuneicephala is a circular molecule of 16,571 bp in length (GenBank accession number KY945352) and contains 13 protein-coding genes (PCGs), 22 tRNA genes, 2 rRNA genes (12s rRNA and 16s rRNA), and a putative control region. The gene order and organization were consistent with those observed in other Triplophysa fishes (Jing et al. Citation2016; Wang et al. Citation2016). The overall base composition of the mitogenome was 27.60% for A, 29.04% for T, 24.59% for C, and 18.77% for G, with the A + T contents of 56.12%. All 13 PCGs employed ATG as the start codon, except for COI which utilized GTG as the start codon. Four stop codons were observed in the PCGs, including TAA for six genes, TAG for ND1, TA for ND4, and T for five genes, which was similar to that detected in other Triplophysa fishes (Wang et al. Citation2016; Feng et al. Citation2019).
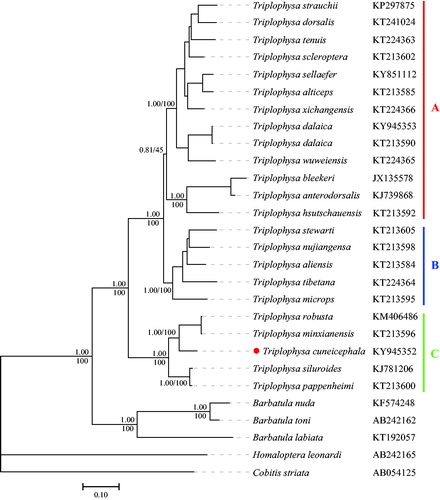
The phylogenetic relationship was reconstructed using Bayesian inference (BI) and maximum-likelihood (ML) methods by MrBayes (Ronquist and Huelsenbeck Citation2003) and RAxML (Stamatakis Citation2006) based on the concatenated protein-coding gene sequences. Both ML and BI phylogenetic tree showed an identical topology with high support values (). Three major clades (A, B and C) were identified among Triplophysa fishes. Interestingly, T. cuneicephala and another Triplophysa species (T. sellaefer) sampled from the same river were clustered into different clades (A and C). T. cuneicephala is closely related to T. robusta and T. minxianensis which are mainly distributed in the upper reaches of the Yellow River.
Figure 1. Phylogenetic tree of Triplophysa species based on 13 concatenated protein-coding genes. The upper and lower numbers at each node represent Bayesian posterior probability and maximum likelihood bootstrap value, respectively. The GenBank accession number of each species was shown on the right of its name.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Chen S, Ya N, Xie C, Wang S, Ren D. 2016. Complete mitochondrial genome of the Triplophysa (Hedinichthys) yarkandensis (Day). Mitochondrial DNA B. 1:235–236.
- Feng X, He D, Sui X, Chen Y, Chen Y. 2018. Morphological and genetic divergence between lake and river populations of Triplophysa in Ngangtse Co, Tibet. Mitochondrial DNA A. 29:778–784.
- Feng X, Chen Y, Sui X, Chen Y. 2019. The complete mitochondrial genome of Triplophysa sellaefer (Cypriniformes: Balitoridae). Mitochondrial DNA B. 4:536–537.
- Jing H, Yan P, Li W, Li X, Song Z. 2016. The complete mitochondrial genome of Triplophysa lixianensis (Teleostei: Cypriniformes: Balitoridae) with phylogenetic consideration. Biochem Syst Ecol. 66:254–264.
- Li J, Si S, Guo R, Wang Y, Song Z. 2013. Complete mitochondrial genome of the stone loach, Triplophysa stoliczkae (Teleostei: Cypriniformes: Balitoridae). Mitochondrial DNA. 24:8–10.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
- Taggart JB, Hynes RA, Prodöuhl PA, Ferguson A. 1992. A simplified protocol for routine total DNA isolation from salmonid fishes. J Fish Biol. 40:963–965.
- Wang Y, Shen Y, Feng C, Zhao K, Song Z, Zhang Y, Yang L, He S. 2016. Mitogenomic perspectives on the origin of Tibetan loaches and their adaptation to high altitude. Sci Rep. 6:29690.
- Wu Y, Wu C. 1992. The fishes of the Qinghai-Xizang plateau. Chendu: Sichuan Publishing House of Science & Technology.
- Zhu SQ. 1989. The loaches of the subfamily Nemacheilinae in China (Cypriniformes: Cobitidae). Nanjing: Jiangsu Science and Technology Press; p. 68–132.
