Abstract
Reboulia hemisphaerica (L.) Raddi is a member of family Aytoniaceae, the third basal group in order Marchantales. Here, we presented complete chloroplast genome of R. hemisphaerica (Genbank accession is MK477551) which is 122,596 bp long and has four subregions: 82,421 bp of large single copy (LSC) and 19,951 bp of small single copy (SSC) regions are separated by 10,112 bp of inverted repeat (IR) regions including 132 genes (87 protein-coding genes, eight rRNAs, and 36 tRNAs). The overall GC content of the chloroplast genome is 28.8% and those in the LSC, SSC, and IR regions are 26.4%, 24.6%, and 42.7%, respectively. Phylogenetic trees with ten chloroplast genomes show phylogenic position of R. hemisphaerica clearly which is clustered with Dumortiera hirsuta. The chloroplast genome will provide high-resolution phylogeny of liverworts in near future.
The thallose liverwort Reboulia hemisphaerica (L.) Raddi, the only species in genus Reboulia and belonging to family Aytoniaceae (Söderström et al. Citation2016), is widely distributed in temperate regions (Boisselier-Dubayle et al. Citation1998). It is characterized by metallic-purple ventral scales with two filiform appendages and star-shaped female receptacles with 3-5 rounded lobes (Casas Citation2009). Various species existed in genus Reboulia; however, Boisselier-Dubayle et al. (Citation1998) they were merged one species back, demonstrating that variance of morphological differences does not always coincide with genetic differences (Hicks Citation1992; Oh and Lee Citation2003; Lee et al. Citation2016): Reboulia queenslandica (Hicks Citation1992) considered as the second species for a while was confirmed as a polyploid cross between two varieties of R. hemisphaerica (Boisselier-Dubayle et al. Citation1998). The family Aytoniaceae, covering five genera including Reboulia, is third basal group in order Marchantiales next to Marchantiaceae and Dumortieraceae (Villarreal et al. Citation2016). To investigate and understand phylogeny of chloroplast genomes in Marchantiales, we completed the chloroplast genome of R. hemisphaerica after those of Marchantia polymorpha subsp. ruderalis (Bowman et al. Citation2017) and Dumortiera hirsuta (Kwon et al. 2019).
The thallus of R. hemisphaerica was collected in Jeongleung-cheon in Seoul, Korea (Voucher in InfoBoss Cyber Herbarium (IN); W. Kwon, IB-50003) and its DNA was extracted by using a DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany). Genome sequencing was performed using HiSeqX at Macrogen Inc., Korea, and de novo assembly and conformation were done by Velvet 1.2.10 (Zerbino and Birney Citation2008), SOAPGapCloser 1.12 (Zhao et al. Citation2011), BWA 0.7.17 (Li Citation2013), and SAMtools 1.9 (Li et al. Citation2009). Geneious R11 11.0.5 (Biomatters Ltd., Auckland, New Zealand) was used for chloroplast genome annotation based on M. polymorpha subsp. ruderalis chloroplast genome (NC_037507).
The chloroplast genome of R. hemisphaerica (Genbank accession is MK477551) is 122,596 bp (GC ratio is 28.8%) and has four subregions: 82,421 bp of large single copy (LSC; 26.4%) and 19,951 bp of small single copy (SSC; 24.6%) regions are separated by 10,112 bp of inverted repeat (IR; 42.7%). It contains 132 genes (87 protein-coding genes, eight rRNAs, and 36 tRNAs); nine genes (four rRNAs and five tRNAs) are duplicated in IR regions.
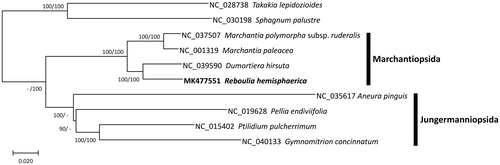
Ten complete chloroplast genomes including R. hemisphaerica were used for constructing neighbor joining (bootstrap repeat is 10,000) and maximum likelihood (bootstrap repeat is 1,000) phylogenic trees using MEGA X (Kumar et al. Citation2018) and IQ-TREE 1.6.6 (Nguyen et al. Citation2014), respectively, after aligning chloroplast genomes using MAFFT 7.388 (Katoh and Standley Citation2013). Phylogenetic trees show clear distinction between two classes Marchantiopsida and Jungermanniopsida as well as R. hemisphaerica and D. hirsuta were clustered in the same clade (), agreeing with that of previous phylogeny studies based on eleven nuclear and organelle DNA markers (Villarreal et al. Citation2016). Once more chloroplast genomes in Marchantiopsida are available, its phylogenetic position will be clearly positioned as third basal group. Our chloroplast genome will be a one of important resources for understanding high-resolution phylogeny of liverworts in near future.
Figure 1. Neighbor joining (bootstrap repeat is 10,000) and maximum likelihood (bootstrap repeat is 1,000) phylogenetic trees of ten complete chloroplast genomes: Reboulia hemisphaerica (MK477551; this study), Marchantia polymorpha subsp. ruderalis (NC_037507), Marchantia paleacea (NC_001319), Dumortiera hirsuta (NC_039590), Pellia endiviifolia (NC_019628), Aneura pinguis (NC_035617), Gymnomitrion concinnatum (NC_040133), Ptilidium pulcherrimum (NC_015402), and two species as an outgroup: Takakia lepidozioides (NC_028738) and Sphagnum palustre (NC_030198). Black bars indicate specific clades with labels. Phylogenetic tree was drawn based on neighbor joining tree. The numbers above branches indicate bootstrap support values of neighbor joining and maximum likelihood phylogenetic trees, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Boisselier-Dubayle M-C, Lambourdiere J, Bischler H. 1998. Taxa delimitation in Reboulia investigated with morphological, cytological, and isozyme markers. Bryologist. 101:61–69.
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y Berger F, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell. e215.171:287-304.
- Casas C. 2009. Handbook of liverworts and hornworts of the Iberian Peninsula and the Balearic Islands: illustrated keys to genera and species. Institut d'Estudis Catalans.
- Hicks ML. 1992. Queensland liverworts–Reboulia Raddi. J Hattori Bot Lab. 71:113–117.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Lee D-H, Park J-S, Choi B-H. 2016. A taxonomic review of Korean Leontopodium R. Br. ex Cassini (Asteraceae). Korean J Pl Taxon. 46:149–162.
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:13033997.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079.
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular biology and evolution. 32:268–274.
- Oh YC, Lee CS. 2003. A taxonomic study on genus Rhynchospora Vahl in Korea. Kor J Plant Tax. 33:393–409.
- Söderström L, Hagborg A, von Konrat M, Bartholomew-Began S, Bell D, Briscoe L, Brown E, Cargill DC, Costa DP, Crandall-Stotler BJ, et al. 2016. World checklist of hornworts and liverworts. PhytoKeys. 59:1.
- Villarreal AJC, Crandall-Stotler BJ, Hart ML, Long DG, Forrest LL. 2016. Divergence times and the evolution of morphological complexity in an early land plant lineage (Marchantiopsida) with a slow molecular rate. New Phytol. 209:1734–1746.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhao Q-Y, Wang Y, Kong Y-M, Luo D, Li X, Hao P. 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinformatics. 12:S2.
