Abstract
The viviparous Multiocellated Racerunner, Eremias multiocellata, is widespread in North China, Mongolia, and the Tuva Republic of Russia. A nearly complete mitochondrial genome of one individual from the suburb of Jingyuan County in Northwest China was determined by next-generation sequencing. The mitogenome is 17,333 bp in size, comprising 2 ribosomal RNA genes, 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), and one control region. The gene arrangement and composition is similar to the typical mitochondrial DNA of vertebrates. With exception to the control region, all of the 37 genes were completely recovered. The concatenated PCGs were used to conduct Bayesian phylogenetic analyses together with several related lizards with mitogenome data in GenBank. The resulting phylogenetic tree confirmed the monophyly of both genus Eremias and its viviparous group. The mitogenome sequence will provide fundamental data for resolving phylogenetic and genetic problems related to Eremias viviparity.
The viviparous Multiocellated Racerunner, Eremias multiocellata Günther, 1872, is widely distributed in North China, Mongolia and the Tuva Republic of Russia (Sindaco and Jeremčenko Citation2008). The advancement of sequencing technology including the next-generation sequencing (NGS) has facilitated rapid obtainment of mitochondrial genome of animals from various groups (Hahn et al. Citation2013). In this study, we determined a nearly complete mitochondrial genome of E. multiocellata by using NGS reads through Roche 454 sequencing platform. The lizard was collected from Jingyuan County (N36.55°, E104.68°), Gansu Province, China. The specimen with field number Guo2760 was deposited in the herpetological collection at Chengdu Institute of Biology, Chinese Academy of Sciences. We took the similar strategy as described previously (Chen et al. Citation2019) to assemble and annotate the mitogenome, by using a published sequence of E. multiocellata (GenBank accession number KM257724; Tong et al. Citation2016) as queries for the reference. A nearly complete mitogenome of 17,333 bp was obtained and deposited in GenBank with accession number MK261077.
The nearly complete mtDNA sequence consists of two ribosomal RNA genes, 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs) and a control region (CR or D-loop). The gene organization and order exhibited a typical vertebrate mitochondrial genome feature. The majority of genes in the mtDNA of E. multiocellata was distributed on H-strand, except for the ND6 gene and eight tRNAs (tRNA-Gln, Ala, Asn, Cys, Tyr, Ser[UGA], Glu, and Pro). In 13 PCGs, the shortest one was ATP8 gene (162 bp) and the longest one was ND5 gene (1824 bp). Twelve of 13 PCGs were initiated with the typical ATG codon, except for COI with GTG. Meanwhile, ND1, ATP8, ATP6, ND4L, ND5, and Cytb genes were terminated with TAA as stop codon; COI and ND6 genes end with AGG, and the remaining five PCGs end with the incomplete termination codon T. The 22 tRNA genes ranged in size from 62 bp in tRNA-Cys to 73 bp in five tRNAs (tRNA-Phe, Leu, Asn, Asp, and Glu). The 12S and 16S rRNA genes were 952 bp and 1545 bp in size, respectively. As for the D-loop, 1767 bp were already determined adjacent to tRNA-Pro, albeit with only 156 bp prior to tRNA-Phe.
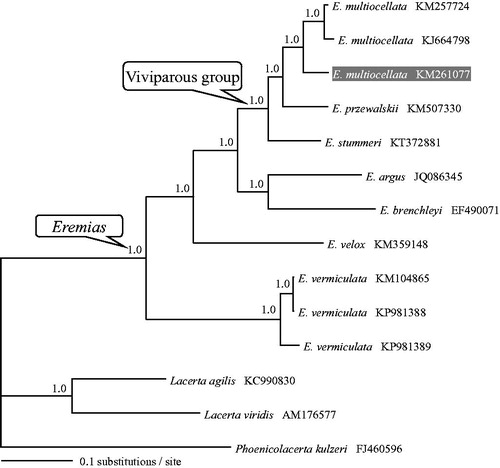
The concatenated PCGs of Eremias available in GenBank plus three lacertids as outgroups were used to reconstruct the Bayesian phylogenetic trees for assessing mitochondrial sequence authenticity of E. multiocellata and its phylogenetic placement. As shown in , in support of Guo et al. (Citation2011), the monophyly of both Eremias and the viviparous group were recovered. Three individuals of E. multiocellta clustered together and were closely related to E. przewalskii. The newly determined mitogenome sequence will provide fundamental data for resolving phylogenetic and genetic problems related to viviparity of Eremias.
Figure 1. A majority-rule consensus tree inferred from Bayesian inference using MrBayes v.3.2.2 (Ronquist et al. Citation2012) under the GTRGAMMA model, based on the concatenated PCGs of 11 individuals of racerunner lizards and three outgroups. The novel sequencing sample is highlighted. GenBank accession numbers are given with species names. DNA sequences were aligned in MEGA v.6.06 (Tamura et al. Citation2013). The PCGs were translated to amino acids sequences, and were manually concatenated all sequences into a single nucleotide dataset (in total 11,393 bp). Node numbers show Bayesian posterior probabilities. Branch lengths represent means of the posterior distribution.
Acknowledgments
The first author wishes to thank Dr. Yun Xia (Chengdu Institute of Biology, Chinese Academy of Sciences) for helping with mitogenome assembly.
Disclosure statement
No potential conflict of interest was reported by the authors. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Chen D, Li J, Guo X. 2019. Next-generation sequencing yields a nearly complete mitochondrial genome of the Forsyth’s toad-headed agama, Phrynocephalus forsythii (Reptilia, Squamata, Agamidae). Mitochondrial DNA Part B. 41:817–819.
- Guo X, Dai X, Chen D, Papenfuss TJ, Ananjeva NB, Melnikov DA, Wang Y. 2011. Phylogeny and divergence times of some racerunner lizards (Lacertidae: Eremias) inferred from mitochondrial 16S rRNA gene segments. Mol Phylogenet Evol. 61:400–412.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads-a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–542.
- Sindaco R, Jeremčenko VK. 2008. The Reptiles of the western Palearctic. Vol. 1. Annotated checklist and distributional atlas of the turtles, crocodiles, amphisbaenians and lizards of Europe, North Africa, Middle East and Central Asia. Latina, Italy: Edizioni Belvedere.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Tong Q, Yao YT, Lin LH, Ji X. 2016. The complete mitochondrial genome of Eremias multiocellata (Squamata: Lacertidae). Mitochondrial DNA Part A. 27:1654–1655.
