Abstract
The complete mitochondrial genome of the Japanese pond turtle Mauremys japonica from Japan was analyzed using next-generation sequencing. The mitochondrial genome of M. japonica was identified as a 16,443 bp circular molecule containing 13 protein-coding genes (PCGs), 22 tRNA genes, and 2 rRNA genes, along with 1 A + T-rich control region; the average AT content was 62.82%. One extra base was present at the 174th position in the ND3 gene. The phylogenetic analyses of the complete mitochondrial DNA genes strongly supported the result obtained from the phylogenetic analysis of partial DNA sequences, grouping the monophyletic species within the genus Mauremys.
Japanese pond turtles (Mauremys japonica) inhabit Honshu, Shikoku, Kyushu and some remote islands of Japan (Suzuki and Hikida Citation2011). An endemic species, M. japonica has been listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and has been designated as a near threatened species according to the Ministry of the Environment, Japan (Yasukawa et al. Citation2008). Here, we report the complete mitochondrial genome of the endemic Japanese turtle M. japonica.
DNA samples from oral cells of M. japonica found in ponds in Kyoto City, Japan (35°04′08.8″N, 135°45′23.8″E), were immediately extracted using DNeasy mini kit (QIAGEN) for mitochondrial DNA analysis. The specimen was stored in a freezer at −20 °C in our laboratory at the Kyoto Sangyo University (Japan). Genomic DNA isolated from oral cells was sequenced using Illumina’s MiSeq platform (Illumina). The resultant reads were assembled and annotated using the MITOS web server (Bernt et al. Citation2013) and Geneious R9 (Biomatters) (Kearse et al. Citation2012). Phylogenetic analysis was performed using TREEFINDER (Jobb Citation2015) based on the nucleotide sequences of the 13 protein-coding genes (PCGs).
We succeeded in sequencing the entire mitochondrial genome of M. japonica from Kyoto (DDBJ accession number AP019397). The genome consisted of a closed loop 16,443 bp long, which included 13 PCGs, 22 tRNA genes, 2 rRNA genes, and 1 AT-rich control region, which represents a typical turtle mitochondrial genome (Shin et al. Citation2015; Zhao, Li, et al. Citation2016; Zhao, Zhang, et al. Citation2016; Feng et al. Citation2017; Li et al. Citation2017; Wang et al. Citation2017). The average AT content of the M. japonica mitochondrial genome was 62.82%. The heavy strand was predicted to have 12 protein-coding and 14 tRNA genes; while the light strand was predicted to contain one protein-coding, eight tRNA, and two rRNA genes. All PCGs began with ATG as the start codon, except COI, which had GTG as the start codon. Stop codons were variable for all PCGs: six genes (COII, ATP8, ATP6, ND4L, ND4, and ND5) used TAA; and COI and ND6 used AGG; and ND1, ND2, and ND3 used TAG as the stop codon. Incomplete stop codons TA (found in COIII, and Cytb) were identified. One extra base was present at the 174th position in the ND3 gene. This was similar to the results of turtles in previous studies (Mindell et al. Citation1998).
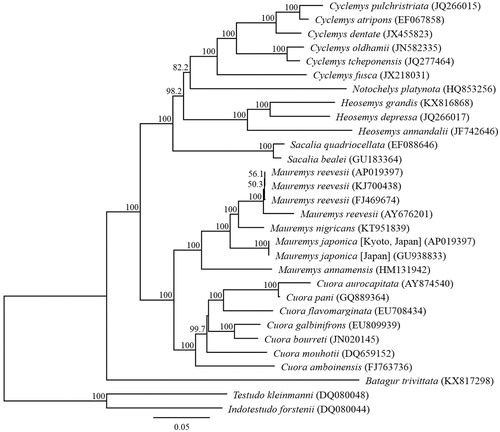
Phylogenetic analysis was performed using the sequences of 13 PCGs and the corresponding ones of 30 closely related taxa (). The phylogenetic analyses of the complete mitochondrial DNA genes strongly supported the result obtained from the phylogenetic analysis of partial DNA sequences, grouping the monophyletic species within the genus Mauremys (Honda et al. Citation2002; Suzuki and Hikida Citation2011; Guillon et al. Citation2012; Suzuki et al. Citation2014). The phylogenetic relationship was consistent with that reported in previous studies, which predicted a sister relationship between M. japonica and M. nigricans. The complete sequence of the M. japonica mitochondrial genome provides additional genetic tools for studying the conservation genetics and biogeography of this species.
Figure 1. Phylogenetic relationships (maximum likelihood) of pond turtles based on nucleotide sequences of the 13 protein-coding genes of the mitochondrial genome. The numbers at the nodes indicate bootstrap support inferred from 1000 bootstrap replicates. Alphanumeric terms in parentheses indicate the DNA Database of Japan accession numbers.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Feng L, Yang J, Zhang YP, Zhao GF. 2017. The complete mitochondrial genome of the Burmese roofed turtle (Batagur trivittata) (Testudines: Geoemydidae). Conservation Genet Resour. 9:95–97.
- Guillon JM, Guéry L, Hulin V, Girondot M. 2012. A large phylogeny of turtles (Testudines) using molecular data. Contributions Zool. 81:147–158.
- Honda M, Yasukawa Y, Hirayama R, Ota H. 2002. Phylogenetic relationships of the Asian box turtles of the genus Cuora sensu lato (Reptilia: Bataguridae) inferred from Mitochondrial DNA sequences. Zool. Sci. 19:1305–1312.
- Jobb G. 2011. TREEFINDER version of March 2011. Munich. http://www.treefnder.de.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649.
- Li J, Lu Y, Zan J, Nie L. 2017. Complete mitochondrial genome of the Cyclemys pulchristriata (Chelonia: Geoemydidae). Mitochondrial DNA Part B. 2:403–404.
- Mindell DP, Michael D, Sorenson MD, Dimcheff DE. 1998. An extra nucleotide is not translated in mitochondrial ND3 of some birds and turtles. Mol Biol Evol. 15:1568–1571.
- Shin HW, Jang KH, Ryu SH, Choi HE, Hwang UW. 2015. Complete mitochondrial genome of the Korean reeves’s turtle Mauremys reevesii (Reptilia, Testudines, Geoemydidae). Mitochondrial DNA Part A. 26:676–677.
- Suzuki D, Hikida T. 2011. Mitochondrial phylogeography of the Japanese pond turtle, Mauremys japonica (Testudines, Geoemydidae). J Zool Syst Evol Res. 49:141–147.
- Suzuki D, Yabe T, Hikida T. 2014. Hybridization between Mauremys japonica and Mauremys reevesii inferred by Nuclear and Mitochondrial DNA Analyses. J Herpetology. 48:445–454.
- Wang Y, Dai X, Wang M, Nie L. 2017. The complete mitochondrial genome of the Heosemys depressa (Testudines, Geoemydidae). Mitochondrial DNA Part B. 2:437–438.
- Yasukawa Y, Yabe T, Ota H. 2008. Population structure and growth of the Japanese pond turtle, Mauremys japonica. In: Rhoin AGJ, Pritchard PCH, van Dijk, Saumure RA, Buhlmann KA, Iverson JB. editors. Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group. Chelonian Research Monographs No5. Luneburg, MA: Chelonian Research Foundation; p. 003.1–003.6.
- Zhao J, Zhang X, Li W, Zhang D, Zhu X. 2016. The complete mitochondrial genome of the endangered Chinese black-necked pond turtle, Mauremys nigricans. Mitochondrial DNA Part B. 1:64–65.
- Zhao J, Li W, Zhang D, Wen P, Zhu X. 2016. The mitochondrial genomes of three lineages of Asian yellow pond turtle, Mauremys mutica. Mitochondrial DNA Part A. 27:2466–2467.
