Abstract
Betula alnoides is a fast-growing hardwood species in South-east Asia and South China. In this study, the complete chloroplast genome (cpDNA) sequence of B. alnoides was determined from Illumina HiSeq pair-end sequencing data. The cpDNA is 161,022 bp in length, contains a large single-copy region (LSC) of 89,440 bp and a small single-copy region (SSC) of 19,535 bp, which were separated by a pair of inverted repeat (IR) regions of 26,022 bp. The genome contains 134 genes, including 86 protein-coding genes, 8 ribosomal RNA genes, and 41transfer RNA genes. The overall GC content of the whole genome is 36.0%, and the corresponding values of the LSC, SSC, and IR regions are 33.6%, 29.4%, and 42.5%, respectively. Further, phylogenomic analysis showed that B. alnoides clustered in a unique clade in genus Betula.
Betula alnoides is a valuable hardwood species in South-east Asia and South China (Zeng et al. Citation2003). It is important wood for furniture manufacture and room decoration in South China (Chen et al. Citation2018). Betula alnoides has been used in large-scale plantation development in tropical and warm subtropical areas in South China and Southeast Asia (Wang Citation2006). However, there has been no genomic studies on B. alnoides.
Herein, we reported and characterized the complete B. alnoides plastid genome (MK610319). One B. alnoides individual (specimen number: 2018070520, deposited at Yunnan Academy of Forestry) was collected from Jinghong, Yunnan Province of China (24°24′25″ N, 101°59′21″ E). DNA was extracted from its fresh leaves using DNA Plantzol Reagent (Invitrogen, Carlsbad, CA, USA).
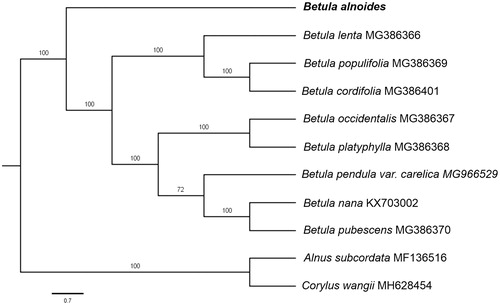
Paired-end reads were sequenced using Illumina HiSeq system (Illumina, San Diego, CA). In total, about 22.4 million high-quality clean reads were generated with adaptors trimmed. Aligning, assembly, and annotation were conducted by CLC de novo assembler (CLC Bio, Aarhus, Denmark), BLAST, GeSeq (Tillich et al. Citation2017), and GENEIOUS v 11.0.5 (Biomatters Ltd, Auckland, New Zealand). To confirm the phylogenetic position of B. alnoides, other eight species of genus Betula from NCBI were aligned using MAFFT v.7 (Katoh and Standley Citation2013) and maximum likelihood (ML) bootstrap analysis was conducted using RAxML (Stamatakis Citation2006); bootstrap probability values were calculated from 1000 replicates. Alnus subcordata (MF136516) and Corylus wangii (MH628454) were served as the out-group.
The complete B. alnoides plastid genome is a circular DNA molecule with the length of 161,022 bp, contains a large single-copy region (LSC) of 89,440 bp and a small single-copy region (SSC) of 19,535 bp, which were separated by a pair of inverted repeat (IR) regions of 26,022 bp. The overall GC content of the whole genome is 36.0%, and the corresponding values of the LSC, SSC, and IR regions are 33.6%, 29.4%, and 42.5%, respectively. The plastid genome contained 134 genes, including 86 protein-coding genes, 8 ribosomal RNA genes, and 41transfer RNA genes. Phylogenetic analysis showed that B. alnoides clustered in a unique clade in genus Betula (). The determination of the complete plastid genome sequences provided new molecular data to illuminate the Betula evolution.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen L, Wang C, Dell B, Zhao Z, Guo J, Xu D, Zeng J. 2018. Growth and nutrient dynamics of Betula alnoidess seedlings under exponential fertilization. J Forest Res. 1:111–119.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes . Nucleic Acids Res. 45:W6–W11.
- Wang WB. 2006. A study on community characteristics of Betula alnoidess plantation. J West China Forest Sci. 3:8–13.
- Zeng J, Zou Y, Bai J, Zheng H. 2003. RAPD analysis of genetic variation in natural populations of Betula alnoidess from Guangxi, China. Euphytica. 1:33–41.