Abstract
Lake Lanao, the second largest lake in the Philippines and one of the 15 ancient lakes in the world, used to contain 20 endemic cyprinid species, which had attracted the attention of evolutionary biologists in the past. Over the years, there has been a steady decline in the abundance and diversity of endemic fishes in the lake because of overfishing and introduction of non-native species. This study represents the first molecular survey of the ichthyofauna of Lake Lanao. A total of 75 specimens of 12 different species belonging to nine genera, eight families, and five orders were DNA barcoded using the mitochondrial cytochrome c oxidase subunit I (COI) gene. Average Kimura 2-parameter genetic distances were 0.24% (within species), 8.31% (between species), 9.69% (within family), and 24.86% (between families). Possible hybrids between Oreochromis species were detected. Glossogobius spp. was highlighted for further taxonomic investigation because barcoding indicated unidentified species of this genus. The partial sequence of mitochondrial COI gene was found to be a good DNA barcode for fast and accurate species identification of fishes in Lake Lanao and for tagging species that warrant further taxonomic investigation.
Introduction
Lake Lanao watershed was considered an important biodiversity site in the Philippines. The lake has an area of about 35,250 ha, a maximum depth of 112 m and a mean depth of 60.2 m (Frey Citation1969). It is located in the province of Lanao del Sur in Mindanao. It is estimated to be between 5.3 and 5.6 million years old (Ismail et al. Citation2014) and is considered one of the 15 ancient lakes in the world. The lake was once home to 20 endemic cyprinid species belonging to the genera Cephalakompsus, Mandibularca, Ospatulus, and Barbodes. This endemic fauna played an important role in the development of species flock concept that had been widely cited in discussions on evolutionary rates (Herre Citation1933; Myers Citation1960; Kornfield and Carpenter Citation1984). Because of overexploitation and introduction of non-native species, there are now only two endemic species remaining in the waters of Lake Lanao, namely, Barbodes lindog and B. tumba (Ismail et al. Citation2014). Owing to the multiple introductions of non-resident species to Lake Lanao, the natural diversity of fish species in the lake has become uncertain. Ismail et al. (Citation2014) reported that there is a continuing decline in the abundance and diversity of native and endemic fish species in Lake Lanao.
Currently, there are plans to restore and conserve the lake fish fauna. However, every fish management and conservation effort requires accurate identification of species. In some cases, identification using morphological characters can be problematic and tedious. Thus, Hebert et al. (Citation2003) proposed a standard gene sequence, the mitochondrial DNA cytochrome c oxidase subunit I (COI) gene, for fast and accurate identification and differentiation of animal species. This study aimed to identify and catalogue the remaining ichthyofauna of Lake Lanao through the use of DNA barcoding.
Materials and methods
Five to 10 individuals of each species were obtained from fishermen and from fish landing centers in Padian, Marawi City. Fish were identified according to the methods described by Herre (Citation1924a, Citation1924b, Citation1926, Citation1932), Trewavas (Citation1942, Citation1982), Akihito and Meguro (Citation1975), Conlu (Citation1986), Teugels et al. (Citation1999), Hoese and Allen (Citation2009, Citation2011), Moyle (Citation2002), Low and Lim (Citation2012), and Froese and Pauly (Citation2017). Current valid scientific names were verified according to the method described by Eschmeyer et al. (Citation2017). The fish samples were fixed and stored in 10% formalin and deposited as voucher specimens at the Institute of Biology, University of the Philippines, Diliman, Quezon City, Philippines.
About 20 mg of muscle tissue was subjected to DNA extraction using either QIAGEN DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA) following the manufacturer’s protocol or InstaGene Matrix (Bio-Rad Laboratories) following the protocol of Hoff-Olsen et al. (Citation1999). Combinations of forward and reverse primers designed by Ward et al. (Citation2005) were used to amplify the COI gene. Polymerase chain reactions (PCR) were done in 50-μL volumes. The PCR mix consisted of 1.0 μL of dNTP (0.05 mM), 2.5 μL of each primer (0.1 mM), 5.0 μL of PCR buffer, 0.5 μL (1.25 U) of Taq polymerase (Roche Taq dNTPack or Vivantis), 34.5 μL of ultrapure water, and 4.0 μL of DNA template (20–100 ng/μL). The PCR conditions followed Ward et al. (Citation2005) except for Glossogobius spp. in which the PCR mix was added with 5% DMSO and 5% MgCl2, the PCR annealing temperature was set at 50 °C for 30 s, and the number of cycles was increased to 40. PCR products were analysed by electrophoresis using 1% agarose gel with ethidium bromide. Approximately 650 bp-sized bands were excised from the gel and purified using QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA) following the manufacturer’s protocol. Purified DNA products were sent to 1st BASE Laboratories Sdn Bhd, Selangur, Malaysia, for bidirectional sequencing.
The DNA sequences were assembled and trimmed using Staden Package4 (Staden et al. Citation2000). MEGA 7 (Kumar et al. Citation2016) was used to align the COI sequences, analyse for genetic divergences based on the Kimura 2-parameter (K2P) model (Kimura Citation1980), and construct a neighbor-joining (NJ) tree at 1000 pseudoreplicates (Saitou and Nei Citation1987). The DNA sequences were submitted to GenBank and were assigned Accession numbers MG407349–MG407423. Trace files, DNA sequences, digital images, and other metadata were submitted to Barcode of Life Data System (BOLD). For species with ambiguous identity, additional COI species sequences available in GenBank and BOLD were downloaded and used for further analyses.
Results
A total of 75 COI sequences were generated from 12 species belonging to nine genera, eight families, and five orders (). Among the 12 species, one is endemic (Barbodes tumba), three are indigenous (Anabas testudineus, Clarias batrachus, and Channa striata), and the rest are introduced to the Philippines. Nine of the species recorded in this study had been previously reported in the lake, while Coptodon zillii, Oreochromis aureus, and Trichopodus trichopterus are new records in the lake.
Figure 1. Unrooted Neighbor-joining tree of 75 cytochrome c oxidase subunit I sequences from 12 Lake Lanao fish species (marked with filled triangles) and 31 reference sequences (obtained from GenBank) using Kimura 2-parameter distances and based on 552 nucleotides. Analyses were conducted using MEGA 7 software (Kumar et al. Citation2016). GenBank accession numbers are given after each scientific name. Numbers on nodes represent percentage bootstrap support out of 1000 bootstrap sampling. Bootstrap support values less than 90% are not shown. The scale bar represents 2 substitutional changes per 100 nucleotide positions. The fishes belong to five orders: Ananbantiformes (Anabantidae, Channidae, Osphronemidae), Cypriniformes (Cyprinidae), Cichliformes (Cichlidae), Gobiiformes (Eleotridae, Gobiidae), and Siluriformes (Clariidae).
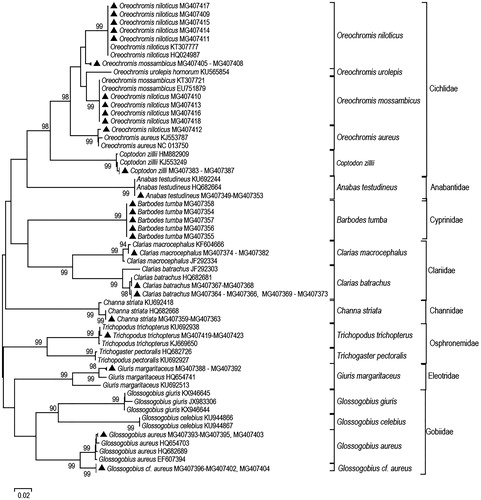
The amplified COI sequences ranged from 652 bp to 655 bp. The mean conspecific, congeneric, and confamilial nucleotide divergences were 0.24%, 8.31%, and 9.69%, respectively. Each individual clustered within its own genus and family (). Except for Glossogobius and Oreochromis specimens, each individual clustered within its presumed species. The 12 Glossogobius sequences generated in this study grouped separately from the G. giuris and G. celebius reference sequences from GenBank. Four of the 12 Glossogobius sequences clustered with the reference sequences of G. aureus. The eight other sequences formed a separate cluster. Each of the two clusters had 99% bootstrap support and each had an average within-cluster K2P distances of 0.29% and 0.0%, respectively. The average pairwise K2P distance between these two clusters is 3.73%. The average pairwise K2P distance of Glossogobius species (collected in this study) from the three reference sequences of G. giuris was 15.87%.
Most of the cichlid specimens collected in this study were morphologically identified as O. niloticus. However, the NJ tree () revealed that some of the O. niloticus-designated specimens clustered with other species. Four specimens from this study were morphologically identified as O. niloticus, but they were molecularly identified as O. mossambicus. This indicates that these four specimens could be hybrids of O. niloticus and O. mossambicus. One specimen from this study is possibly a hybrid between O. niloticus and O. aureus. This specimen is O. niloticus morphologically but molecularly O. aureus as it clustered with that respective species reference. Four specimens morphologically identified as O. mossambicus clustered with the group composed of O. niloticus species in the NJ tree. These four specimens could be hybrids of O. mossambicus and O. niloticus.
Discussion
A total of 36 species of fishes were reported in Lake Lanao (). Only 12 species were recorded in this study, three of which are new records in the lake. Lake Lanao was reported to contain 20 endemic cyprinids. But, in a recent survey, there were only two species, B. tumba and B. lindog, that were found in the lake (Ismail et al. Citation2014). In this study, only one species was collected: B. tumba. A number of reasons are said to be responsible for the decline of endemic cyprinids in the lake: overexploitation, introduction of the predatory fishes Glossogobius spp. and Giuris margaritaceus (Kornfield Citation1982), construction of hydroelectric power plants (Ismail and Escudero Citation2011), climate change, and water pollution.
Table 1. Endemic, native, and introduced fish species reported in Lake Lanao.
Trichopodus pectoralis was the only gourami species that was previously reported from Lanao Lake (Villaluz Citation1966; Escudero Citation1994; Ismail et al. Citation2014). However, another species, T. trichopterus, was recorded in this study. The two species resemble each other; the only difference is the presence of a black blotch in the middle of the flank and another dark spot at the caudal base in T. trichopterus (Low and Lim Citation2012). Despite two years of collecting samples, no specimens of T. pectoralis were obtained in this study.
Three species of Glossogobius have been reported in Lake Lanao: G. aureus (Akihito and Meguro Citation1975), G. giuris (Villaluz Citation1966; Sanguila et al. Citation1975; Kornfield Citation1982; Escudero Citation1994; Ismail et al. Citation2014) and G. celebius (Escudero Citation1994; Ismail et al. Citation2014). Glossogobius giuris and G. aureus are often mistaken for each other because of their high morphological resemblance (Akihito and Meguro Citation1975). Glossogobius giuris was first reported in Lake Lanao by Villaluz (Citation1966). Akihito and Meguro (Citation1975) collected G. aureus specimens from Lake Lanao. Since then, there has been no mention of the presence of G. aureus in the lake in several fish surveys. In this study, G. giuris reference sequences from GenBank grouped separately from the 12 Glossogobius sequences generated in this study. Four of the 12 clustered with the reference sequences of G. aureus; the remaining eight sequences formed a separate cluster. The two clusters had an average pairwise K2P distance of 3.73%, a value that is slightly higher than the threshold of 3.5% set by Hebert et al. (Citation2003) and Ward (2009). This indicates that the cluster consisting of eight sequences could be a different species.
Only two species of tilapia were reported in Lake Lanao in previous studies: O. niloticus and O. mossambicus. In this study, four species of cichlids were detected: O. niloticus (n = 9), O. mossambicus (n = 4), O. aureus (n = 1) and C. zillii (n = 5). There are five species of Oreochromis that were introduced in the Philippines: O. mossambicus, O. niloticus, O. aureus, O. urolepis and O. spirulus (Juliano et al. Citation1989; Guerrero Citation2014). Coptodon zillii was introduced to the Philippines in 1977 (Juliano et al. Citation1989) and could have been introduced into Lake Lanao for aquaculture. Hybridization extensively occurs among Oreochromis species (Nagl et al. Citation2001; Moyle Citation2002). In this study, putative hybrids were detected.
In summary, DNA barcoding using the mitochondrial COI gene was successful in discriminating the 12 species of fishes in Lake Lanao. Three previously unreported species have been noted and the genus Glossogobius has been found to require further taxonomic reassessment. Potential hybridization among Oreochromis species was also detected. Furthermore, this study has updated the ichthyofauna of Lake Lanao. It is hoped that the COI sequences that were submitted to BOLD and GenBank would aid other researchers and policy makers in the accurate identification of fish species in Lake Lanao.
Acknowledgments
The first author would like to acknowledge Mindanao State University, main campus for the APDP grant and the Commission on Higher Education (CHED) for scholarship that was awarded to her. The authors would like to thank Ms. Carima Usman for the assistance extended to us for the collection of some fish species and to Dr. Zubaida U. Basiao, Dr. Ian Kendrich C. Fontanilla, and Dr. Josefa R. Pante for their comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akihito P, Meguro K. 1975. Description of a new gobiid fish, Glossogobius aureus, with notes on related species of the genus. Japan J Ichthyol. 22:127–142.
- Conlu PV. 1986. Guide to Philippine Flora and Fauna. Vol. IX. Fishes. Manila: Natural Resources Management Center, Ministry of Natural Resources and University of the Philippines.
- Eschmeyer WN, Fricke R, van der Laan R. (eds). 2017. Catalog of fishes: genera, species, references; [accessed 2017 July–Aug]. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
- Escudero PE. 1994. Lake Lanao fisheries: problems and recommendations. Philipp Biota. 27:8–18.
- Fowler H. 1941. The fishes of the groups Elasmobranchii, Holocephali, Isospondylii and Ostariophysi obtained by the United States Bureau of Fisheries Steamer Albatross in 1907 to 1910, chiefly in the Philippine islands and adjacent seas. US Natl. Mus. Bull. 100:1–879.
- Fowler HW. 1933. Descriptions of new fishes obtained 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Proc Acad Nat Sci Philadelphia. 85:233–367.
- Frey G. 1969. A Limnological Reconaissance of Lake Lanao. Verh Int Verein Limnol. 17:1090–1102.
- Froese R, Pauly D, editors. 2017. FishBase [accessed 2017 July–Aug]. www.fishbase.org.
- Guerrero RD. 2014. Impacts of introduced freshwater fishes in the Philippines (1905–2013): a review and recommendations. Philipp J Sci. 143:49–59.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proc Biol Sci. 270:313–321.
- Herre A. 1923. A review of the eels of the Philippine archipelago. Philipp J Sci. 23:123–236.
- Herre A. 1924a. Distribution of true fresh-water fishes in the Philippines I. The Philippine Cyprinidae. Philipp J Sci. 24:249–307.
- Herre A. 1924b. Distribution of true fresh-water fishes in the Philippines II. The Philippine Labyrinthici, Clariidae, and Siluridae. Philipp J Sci. 24:683–709.
- Herre A. 1926. Two new fishes from Lake Lanao. Philipp J Sci. 29:499–502.
- Herre A. 1932. Five new Philippine fishes. Copeia. 1932:139–142.
- Herre A. 1933. The fishes of Lake Lanao: a problem in evolution. Am Nat. 67:154–162.
- Hoese DF, Allen GR. 2009. Description of three new species of Glossogobius from Australia and New Guinea. Zootaxa. 1981:1–14.
- Hoese DF, Allen GR. 2011. A review of the amphidromous species of the Glossogobius celebius complex, with description of three new species. Cybium. 35:269–284.
- Hoff-Olsen P, Mevag B, Staalstrom E, Hovde B, Egeland T, Olaisen B. 1999. Extraction of DNA from decomposed human tissue: an evaluation of five extraction methods for short tandem repeat typing. Forensic Sci Int. 105:171–183.
- Ismail GB, Escudero PT. 2011. Threatened fishes of the world: Puntius lindog Herre, 1924 (Cyprinidae). Environ Biol Fishes. 91:117–118.
- Ismail GB, Sampson DB, Noakes DLG. 2014. The status of Lake Lanao endemic cyprinids (Puntius species) and their conservation. Environ Biol Fishes. 97:425–434.
- Juliano RO, Guerrero III R, Ronnquillo I. 1989. The introduction of exotic aquatic species in the Philippines. In: De Silva SS, editor. Exotic aquatic organisms in Asia 1981. Proceedings of the workshop on introduction of exotic aquatic organisms in Asia; June 19–21, Manila, Philippines: Asian Fish Society Special Publication; Vol. 3; p. 83–90.
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111–120.
- Kornfield I. 1982. Report from Mindanao. Copeia. 1982:493–495.
- Kornfield I, Carpenter KE. 1984. Cyprinids of Lake Lanao, Philippines: taxonomic validity, evolutionary rates and speciation scenarios. In: Echelle AE, Kornfield I, editors. Evolution of fish species flocks. Orono: University of Maine Press; p. 69–84.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Low BW, Lim KK. 2012. Gouramies of the genus Trichopodus in Singapore (Actinopterygii: Perciformes: Osphronemidae). Nat Singap. 5:83–93.
- Moyle PB. 2002. Inland fishes of California: revised and expanded. Berkeley (CA): University of California Press.
- Myers GS. 1960. The endemic fish fauna of Lake Lanao, and the evolution of higher taxonomic categories. Evolution. 14:323–333.
- Nagl S, Tichy H, Mayer WE, Samonte IE, McAndrew BJ, Klein J. 2001. Classification and phylogenetic relationships of African tilapiine fishes inferred from mitochondrial DNA sequences. Mol. Phylogenetics Evol. 20:361–374.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Sanguila WM, Rosales A, Tabaranza B, Jr, Mohamad S. 1975. Notes on the food habits of Puntius sirang and Glossogobius giuris. Mindanao J. 1:3–11.
- Staden R, Beal KF, Bonfield JK. 2000. The Staden package, 1998. Methods Mol Biol. 132:115–130.
- Teugels GG, Diego RC, Pouyaud L, Legendre M. 1999. Redescription of Clarias macrocephalus (Siluriformes: Clariidae) from South-east Asia. Cybium. 23:285–295.
- Trewavas E. 1942. XLIV-The cichlid fishes of Syria and Palestine. J Nat Hist. 9:526–536.
- Trewavas E. 1982. Tilapias: taxonomy and speciation. In: Pullin RSV, Lowe-McConnell RH, editors. The biology and culture of tilapias 1982. ICLARM Conference Proceedings 7 of the International Conference on the Biology and Culture of Tilapias; 1980 Sep 2–5; Study and Conference Center of the Rockefeller Foundation, Bellagio, Italy. Manila, Philippines: International Center for Living Aquatic Resources Management; p. 432
- Villaluz DK. 1966. The Lake Lanao fisheries and their conservation. Manila: Bureau of Printing.
- Ward RD. 2009. DNA barcode divergence among species and genera of birds and fishes. Mol Ecol Resour. 9:1077–1085.
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. 2005. DNA barcoding Australia's fish species. Philosophical Translocations R Soc Lond B. 360:1847–1857.
- Wood CE. 1968. Two species of Cyprinidae from north central Mindanao. Philipp J Sci. 95:411–423.
