Abstract
Cephalotus follicularis is a carnivorous plant native to southwest Australia that belongs to the monospecific family Cephalotaceae. It forms both carnivorous pitcher leaves and non-carnivorous flat leaves, the pitcher leave makes it able to attract, catch, and digest their preys, usually insects, and assimilate nutrients for the growth. Previous studies of the plastid genome in carnivorous plants show various evolutionary changes. In this study, we report the complete plastid genome of C. follicularis, the circular plastid genome possesses a total length 142,706 bp with the typical quadripartite structure of angiosperms, contains 100 genes, the whole set of ndh-gene family in C. follicularis have been truncated or pseudogenized. Phylogenetic analyses based on plastid coding genes showed that C. follicularis and Averrhoa carambola formed the clade corresponding to Oxalidales. The complete plastome sequence of C. follicularis will provide a useful resource for the evolutionary biology study of carnivorous plants as well as for the phylogenetic studies in Oxalidales.
Carnivorous plants are able to attract, catch, and digest their preys, usually insects, and assimilate nutrients for the growth (Adamec Citation1997). The obtained nutrients are primarily used as source of nitrogen enabling these plants to survive in oligotrophic environments. Previous studies of organelle genomes of carnivorous plants of Lentibulariaceae revealed the loss and pseudogenization of the NAD(P)H dehydrogenase genes, increased substitution rates and relaxed purifying selection in the plastid genomes (Wicke et al. Citation2014; Silva et al. Citation2016). While the complete plastome of another carnivorous plants, Nepenthes mirabilis contains the typical structure and gene contents of angiosperm plastomes (Zhu et al. Citation2018). In Droseraceae, the plastid genomes show multiple rearrangements, gene losses and large expansions or contractions of the inverted repeats (Nevill et al. Citation2019).
Cephalotus follicularis (Cephalotus), a carnivorous plant native to southwest Australia that belongs to the monospecific family Cephalotaceae in the order Oxalidales, forms both carnivorous pitcher leaves and non-carnivorous flat leaves (Pavlovič Citation2011; Fukushima et al. Citation2017). During the springtime, the plant produces a foliage type of non-carnivorous leaf called lamina. Later, the second type of leaf is produced, carnivorous pitcher.
In this study, we downloaded total compressed 2.4G data of high-quality reads (NCBI accession # DRR053719) using the fastq-dump software (https://ncbi.github.io/sra-tools/fastq-dump.html), which was released by the C. follicularis genome sequencing project (Fukushima et al. Citation2017). The axenically grown plants of C. follicularis for sequencing were obtained from CZ Plants Nursery (Trebovice, Czech Republic) and plantlets vegetatively propagated on half-strength MS medium (Fukushima et al. Citation2017). These reads were then used to assemble the complete plastid genome using the plastid genomes Averrhoa carambola (NC_033350) as reference. We performed the assembling and annotation using Geneious 10.2.2 and adjusted the genes manually to make sure that were maintained as open reading frames. IR boundaries for the draft plastome were confirmed using BLAST. Finally, we obtained a chloroplast genome of C. follicularis and submitted the whole genome to GenBank (MK460223).
The plastome of C. follicularis was found to possess a total length 142,706 bp with the typical quadripartite structure of angiosperms, containing two Inverted Repeats (IRs) of 25,949 bp separated by a Large Single-Copy (LSC) region and a Small Single-Copy (SSC) region of 82,070 and 8378 bp, respectively. The plastid genome of C. follicularis contains 100 genes, including 66 protein-coding genes, four ribosomal RNA genes, and 30 transfer RNA genes. The whole set of ndh-gene family in C. follicularis has been truncated or pseudogenized. The overall GC content of C. follicularis plastid genome is 37.1% and the corresponding values in LSC, SSC, and IR regions are 34.6%, 28.6%, and 42.7%, respectively.
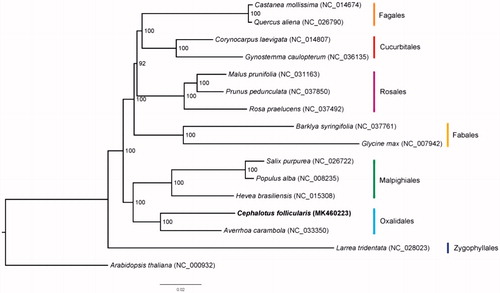
We also used the coding sequences of C. follicularis and another 15 plastomes to reconstruct a Maximum-Likelihood tree through RAxML (Stamatakis Citation2014) under the GTRGAMMA substitution model, with 1000 bootstraps on CIPRES website (Miller et al. Citation2010). The result shows that C. follicularis and Averrhoa carambola formed the clade corresponding to Oxalidales (). The new plastome sequence of C. follicularis reported here will provide a useful resource for the evolutionary biology study of carnivorous plants as well as for the phylogenetic studies in Oxalidales.
Figure 1. Phylogenetic tree based on the coding sequences of 16 plastomes. Bootstrap support values from 1000 replicates are shown above branches. All the plastome sequences are available in GenBank, with the accession numbers listed right to their scientific names. The new plastome obtained in this study is shown in bold.
Authors’ contributions
S.S.W. conceived the study; M.X.C. and Z.H.L obtained the molecular data; X.Y.D., X.Q.W., Y.X.L., M.X.C., and Z.H.L. participated in data analysis; M.X.C. and Z.H.L. drafted the manuscript; S.S.W. revised the manuscript. All authors provided comments and final approval.
Acknowledgments
We are grateful to National Institute for Basic Biology, Okazaki 444-8585, Japan. Department of Basic Biology, School of Life Science, SOKENDAI (Graduate University for Advanced Studies), Okazaki 444-8585, Japan. Department of Biochemistry and Molecular Genetics, University of Colorado School of Medicine, Aurora, Colorado 80045, USA. BGI-Shenzhen, Shenzhen 518083, China. Department of Computer Science, City University of Hong Kong, Hong Kong 999077, China. Department of Biology, University of Nevada, Reno, Nevada 89557, USA. Department of Plant Systems Biology, VIB, Ghent University, Ghent 9052, Belgium. Department of Biological Sciences, University at Buffalo, Buffalo, New York 14260, USA. Department of Biological Sciences, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan. School of Food, Agricultural and Environmental Sciences, Miyagi University, Miyagi 982-0215, Japan. Department of Plant Science, School of Agriculture, Tokai University, Kumamoto 869-1404, Japan. Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Kashiwa 277-8568, Japan. Departament de Genètica and Institut de Recerca de la Biodiversitat (IRBio), Universitat de Barcelona, Diagonal 643, Barcelona 08028, Spain. Center for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, 1350K Copenhagen, Denmark. Graduate School of Bioagricultural Sciences, Nagoya University, Nagoya 464-8601, Japan. 16Advanced Science Research Center, Kanazawa University, Kanazawa 920-0934, Japan. Department of Biological Sciences, Tokyo Metropolitan University, Hachioji 192-0397, Japan. Department of Mathematics and Statistics, University of Ottawa, K1N 6N5 Ottawa, Canada. Tohoku Medical Megabank Organization, Tohoku University, Sendai 980-8573, Japan. Department of Natural Science, Osaka Kyoiku University, Osaka 582-8582, Japan for their support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adamec L. 1997. Mineral nutrition of carnivorous plants: a review. Bot Rev. 63:273–299.
- Fukushima K, Fang X, Alvarez-Ponce D, Cai H, Carretero-Paulet L, Chen C, Chang TH, Farr KM, Fujita T, Hiwatashi Y, et al. 2017. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat Ecol Evol. 1:59.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), Orleans 1–8.
- Nevill PG, Howell KA, Cross AT, Williams AV, Zhong X, Tonti-Filippini J, Boykin LM, Dixon KW, Small I. 2019. Plastome-wide rearrangements and gene losses in carnivorous Droseraceae. Genome Biol Evol. 11:472–485.
- Pavlovič A. 2011. Photosynthetic characterization of Australian pitcher plant Cephalotus follicularis. Photosynthetica. 49:253–258.
- Silva SR, Diaz YC, Penha HA, Pinheiro DG, Fernandes CC, Miranda VF, Michael TP, Varani AM. 2016. The chloroplast genome of Utricularia reniformis sheds light on the evolution of the ndh gene complex of terrestrial carnivorous plants from the Lentibulariaceae family. PLOS One. 11:e0165176
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Wicke S, Schaferhoff B, dePamphilis CW, Muller KF. 2014. Disproportional plastome-wide increase of substitution rates and relaxed purifying selection in genes of carnivorous Lentibulariaceae. Mol Biol Evol. 31:529–545.
- Zhu ZX, Wang JH, Chen CR, Zhao KK, Wang HF. 2018. Complete plastome sequence of Nepenthes mirabilis (Nepenthaceae): a “vulnerable” herb in China. Mitochondrial DNA B. 3:732–733.
