Abstract
Pueraria montana var. lobata is a traditional Chinese herb with various medicinal purposes. In this study, the complete chloroplast genome of the P. montana var. lobata was obtained by using Illumina HiSeq X Ten. The chloroplast genome was 153,411 bp long, which consisted of a large single-copy (LSC) region of 84, 131 bp and a short single-copy (SSC) region of 17,990 bp separated by a pair of inverted repeat (IR) regions of 25,645 bp. The whole chloroplast genome contained 111 gene species including 77 protein-coding genes (PCG), 30 tRNA, and 4 rRNA species, with 19 of them occurring in double copies. Introns were detected in 12 PCG and 6 tRNA species. The overall GC content is 35.4%. Phylogenetic analysis indicated that P. montana var. lobata was relatively close to Pachyrhizus erosus in phylogeny.
Pueraria montana var. lobate is a semi-woody, perennial liana belonging to the family Leguminosae in possession of a variety of beneficial health effects. It was usually used as traditional Chinese medicine with various medicinal purposes (Li Citation2015; Heo et al. Citation2019). The dry root of P. montana var. lobata was full of secondary metabolites such as isoflavonoids (Rong et al. Citation1998; Joung et al. Citation2003), flavones (Kinjo et al. Citation1988), triterpenoids (Arao et al. Citation1995, Citation1997), and dibenzyl butyrolactones (Hirakura et al. Citation1997). It had the efficacy of cytoprotective activity (Heo et al. Citation2019), hepatoprotective effect (Abascal and Yarnell Citation2007; Zhang et al. Citation2016; Sun et al. Citation2018), antineoplastic activity (Zhang et al. Citation2019), antimicrobial activity (Joung et al. Citation2003), anti-tobacco mosaic virus activity (Cui et al. Citation2018), relieving muscles analgesia, reducing fever and antidiarrheal (Li Citation2015), treating diabetes and cardiovascular diseases (Wong et al. Citation2011; Liu et al. Citation2019), and so on. The isoflavonoids are also common constituents in human diets (Joung et al. Citation2003). To facilitate its genetic research and contribute to its utilization, the complete chloroplast genome of the P. montana var. lobate was assembled from the whole genome Illumina sequencing data and phylogenetic analysis was conducted by using the neighbor-joining (NJ) method.
DNA samples were extracted from the fresh leaves collected from a single individual of P. montana var. lobate in Enshi, Hubei Province of China (109°32′23″E, 29°57′33″N) and stored in the Herbarium of Neijiang Normal University (accession number: 20190225PM04). The Illumina sequencing was conducted on Illumina HiSeq X Ten platform by Beijing Novogene Bioinformatics Technology Co., Ltd (Beijing, China). The complete chloroplast genome was assembled using the baiting and iterative mapping approach (Hahn et al. Citation2013). Cajanus cajan (GenBank accession number KU729879) within the subfamily Papilionoideae was used as a reference for assembling and annotation (Kaila et al. Citation2016). The annotated chloroplast DNA (cpDNA) sequence has been deposited into GenBank with the accession number MK820065.
The complete cpDNA of P. montana var. lobate was a circular molecule of 153,411 bp in length including an LSC region of 84,131 bp and an SSC region of 17,990 bp separated by a pair of IR regions of 25,645 bp each. It encodes a total of 111 genes including 77 protein-coding genes, 30 tRNA, and 4 rRNA species, in which 19 of them were with double copies (8 protein-coding genes, 7 tRNA, and 4rRNA genes). Intron–exon structure analysis indicated that 93 (83.8%) genes had no introns, whereas 18 (16.2%) genes (12 PCG and 6 tRNA genes) contained a single intron and 2 PCG (clpP and ycf3) had two introns. The total GC content is 35.4%, while the corresponding values of the LSC, SSC, and IR regions are 32.8%, 28.9%, and 41.9%, respectively.
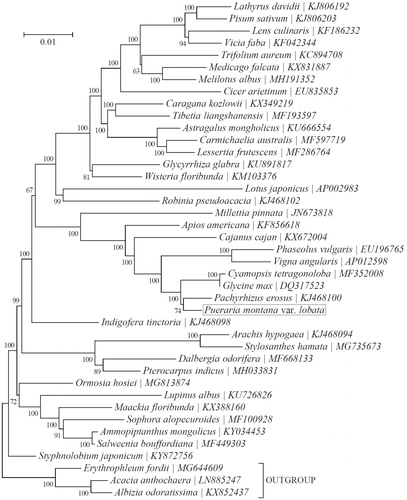
To identify the phylogenetic position of P. montana var. lobata, phylogenetic analysis was conducted using 38 complete chloroplast sequences in Papilionoideae and other 3 species within the subfamily Caesalpinioideae as outgroup taxa by MEGA 7.0 (Kumar et al. Citation2016). The neighbor-joining (NJ) tree indicated that P. montana var. lobata was closely related to Pachyrhizus erosus (). The results will provide a foundation for further investigation of chloroplast genome evolution in Pueraria.
Figure 1. Phylogeny of 38 species within the subfamily Papilionoideae based on the neighbor-joining (NJ) analysis of the concatenated chloroplast protein-coding sequences. Three species within the subfamily Caesalpinioideae were included as outgroup taxa. The position of Pueraria montana var. lobate (GenBank accession number: MK820065) is shown in a box.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abascal K, Yarnell E. 2007. Kudzu – the miracle vine. Alternat Complement Therap. 13:78–85.
- Arao T, Kinjo J, Nohara T, Isobe R. 1995. Oleanene-type triterpene glycosides from Puerariae radix. II. Isolation of saponins and the application of tandem mass spectrometry to their structure determination. Chem Pharma Bull. 7:1176–1179.
- Arao T, Kinjo J, Nohara T, Isobe R. 1997. Oleanene-type triterpene glycosides from Puerariae radix. IV. Six new saponins from Pueraria lobata.Chem Pharma Bull. 2:362–366.
- Cui T, Tang S, Liu C, Li Z, Zhu Q, You J, Si X, Zhang F, He P, Liu Z, et al. 2018. Three new isoflavones from the Pueraria montana var. lobata (Willd.) and their bioactivities. Natural Prod Res. 32:2817–2824.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads – a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129.
- Heo HS, Han GE, Won J, Cho Y, Woo H, Lee JH. 2019. Pueraria montana var. lobata root extract inhibits photoaging on skin through Nrf2 pathway. J Microbiol Biotechnol. 29:518–526.
- Hirakura K, Morita M, Nakajima K, Sugama K, Takagi K, Niitsu K, Ikeya Y, Maruno M, Okada M. 1997. Phenolic glucosides from the root of Pueraria lobata. Phytochemistry. 46:921–928.
- Joung J-y, Mangai Kasthuri G, Park J-y, Kang W-j, Kim H-s, Yoon B-s, Joung H, Jeon J-h. 2003. An overexpression of chalcone reductase of Pueraria montana var. lobata alters biosynthesis of anthocyanin and 5′-deoxyflavonoids in transgenic tobacco. Biochem Biophys Res Commun. 303:326–331.
- Kaila T, Chaduvla PK, Saxena S, Bahadur K, Gahukar SJ, Chaudhury A, Sharma TR, Singh NK, Gaikwad K. 2016. Chloroplast genome sequence of Pigeonpea (Cajanus cajan (L.) Millspaugh) and Cajanus scarabaeoides (L.) Thouars: genome organization and comparison with other legumes. Front Plant Sci. 7:1847.
- Kinjo J-E, Takeshita T, Abe Y, Terada N, Yamashita H, Yamasaki M, Takeuchi K, Murakami K, Tomimatsu T, Nohara T, et al. 1988. Studies on the constituents of Pueraria lobata. IV: chemical constituents in the flowers and the leaves. Chem Pharm Bull. 36:1174–1179.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Li M. 2015. Pueraria lobata (Willd.) Ohwi 葛根 (Gegen, Kudzu)[M]//Dietary Chinese herbs. Vienna: Springer.
- Liu J, Shi YC, Lee DYW. 2019. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin Herbal Med.
- Rong H, Stevens JF, Deinzer ML, Cooman LD, Keukeleire DD. 1998. Identification of isoflavones in the roots of Pueraria lobata. Planta Medica. 7:620–627.
- Sun Y, Zhang H, Cheng M, Cao S, Qiao M, Zhang B, Ding L, Qiu F. 2018. New hepatoprotective isoflavone glucosides from Pueraria lobata (Willd.) Ohwi. Nat Prod Res. 1–8.
- Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. 2011. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 134:584–607.
- Zhang JL, Xu W, Zhou ZR, et al. 2019. Antineoplastic constituents from the chemical diversified extract of Radix puerariae. Chem Biodivers. 1:e1800408.
- Zhang P, Ye Y, Yang X, Jiao Y. 2016. Systematic review on Chinese herbal medicine induced liver injury. Evidence-Based Complement Alternat Med. 2016:3560812.
