Abstract
Rhus typhina, commonly called staghorn sumac, is native to North America and is widely planted as an ornamental deciduous shrub all over the temperate region. In this study, the complete chloroplast genome (plastome) of R. typhina was determined using genome-skimming method. The complete plastome of staghorn sumac was 160,204 bp in length with a canonical quadripartite structure, including a large single-copy region of 87,789 bp, a small single-copy region of 19,319 bp, and a pair of inverted repeats regions of 26,548 bp each. A total of 113 unique genes were annotated in this plastome, including 79 protein-coding genes, 30 tRNA genes, and 4 rRNA genes. The overall guanine-cytosine (GC) content was 37.8%. As shown in the ML phylogenetic tree, R. typhina was closely related to R. chinensis, and Rhus was sister to Pistacia within Anacardiaceae famliy.
Keywords:
Rhus, a genus in the Anacardiaceae family, contains more than 200 species mainly distributed in temperate and subtropical region (Rayne and Mazza Citation2007). R. typhina, commonly called staghorn sumac, is native to North America and is widely planted as an ornamental deciduous shrub all over the temperate region. It was first introduced to China in 1959 (Pan and You Citation1994). For the purpose of viewing and reclamation, staghorn sumac has been planted throughout the Yellow River basin in China. Once established in someplace, each root fragment can develop into a new individual by the way of root suckering, making it very hard to be rooted out (Wang et al. Citation2008). Staghorn sumac is now considered as an invasive species in Shandong Province of China (Wang et al. Citation2008). In this study, we reported the plastome of R. typhina and resolved its phylogenetic position.
Fresh leaves of staghorn sumac were sampled from Lushan Mountain (Shandong, China; 36°21’N, 118°7’E). Voucher specimen (092037B-2) was deposited at College of Life Sciences, Shandong Normal University. Total genomic DNA was extracted by the modified CTAB method described in Qu, Fan, et al. (Citation2019). Therefore, the plastid DNA was not directly extracted (Liu et al. Citation2017). Then total genomic DNA was sequenced using the Illumina Novaseq platform. Plastome assembly was performed by using Organelle Genome Assembler (OGA) described in Qu (Citation2019). Plastome annotation was conducted by using Plastid Genome Annotator (PGA) (Qu Moore, et al. Citation2019), with manual correction using Geneious v9.1.4. The annotated complete plastome was uploaded to GenBank under accession number MT083895.
The complete plastome of staghorn sumac was 160,204 bp in length with a canonical quadripartite structure, including a large single-copy region of 87,789 bp, a small single-copy region of 19,319 bp, and a pair of inverted repeats regions of 26,548 bp each. The overall guanine-cytosine (GC) content was 37.8%. A total of 113 unique genes was annotated in this plastome, including 79 protein-coding genes, 30 tRNA genes, and four rRNA genes were annotated. Among them, ten protein-coding genes (atpF, ndhA, ndhB, petB, petD, rpl2, rpl16, rpoC1, rps12, and rps16) and six tRNA genes (trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA, and trnV-UAC) contained one intron, and two protein-coding genes (clpP and ycf3) contained two introns.
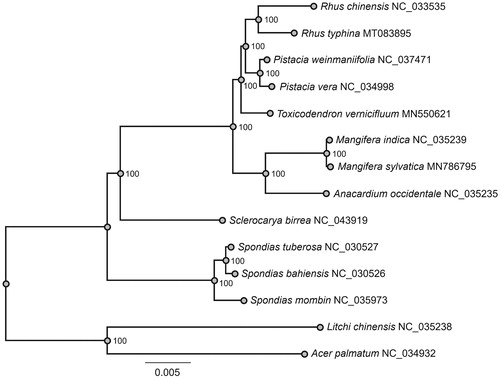
To determine the phylogenetic placement of R. typhina, a maximum likelihood (ML) tree of 14 species () was reconstructed by using RAxML v8.2.10 (Stamatakis Citation2014), based on alignment of 79 shared protein-coding genes by using MAFFT v7.313 (Katoh and Standley Citation2013). As shown in the ML phylogenetic tree, R. typhina was closely related to R. chinensis, and Rhus was sister to Pistacia within Anacardiaceae famliy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol E. 30(4):772–780.
- Liu F, Jin Z, Wang Y, Bi YP, Melton JT. 2017. Plastid genome of Dictyopteris divaricata (Dictyotales, Phaeophyceae): understanding the evolution of plastid genomes in brown algae. Mar Biotechnol. 19(6):627–637.
- Pan ZG, You YT. 1994. Growing exotic trees in China. Beijing (China): Beijing Science and Technology Press.
- Qu X-J. 2019. Complete plastome sequence of an endangered species, Calocedrus rupestris (Cupressaceae). Mitochondrial DNA B. 4(1):762–763.
- Qu XJ, Fan SJ, Wicke S, Yi TS. 2019. Plastome reduction in the only parasitic gymnosperm Parasitaxus is due to losses of photosynthesis but not housekeeping genes and apparently involves the secondary gain of a large inverted repeat. Genome Biol E. 11(10):2789–2796.
- Qu XJ, Moore MJ, Li DZ, Yi TS. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 15(1):50.
- Rayne S, Mazza G. 2007. Biological activities of extracts from sumac (Rhus spp.): a review. Plant Foods Hum Nutr. 62(4):165–175.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Wang G, Jiang G, Yu S, Li Y, Liu H. 2008. Invasion possibility and potential effects of Rhus typhina on Beijing municipality. J Integr Plant Biol. 50(5):522–530.