Abstract
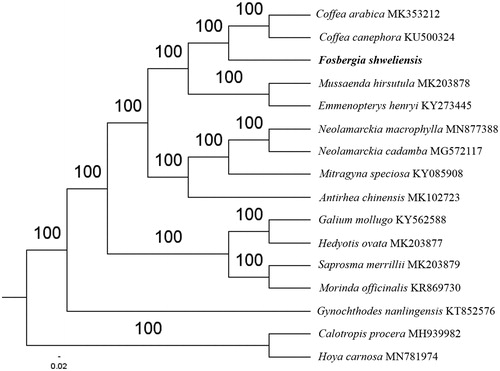
The first complete chloroplast genome (cpDNA) sequence of Fosbergia shweliensis was determined from Illumina HiSeq pair-end sequencing data in this study. The cpDNA is 154,717 bp in length, contains a large single-copy region (LSC) of 84,747 bp and a small single-copy region (SSC) of 18,230 bp, which were separated by a pair of inverted repeats (IR) regions of 25,870 bp. The genome contains 130 genes, including 85 protein-coding genes, 8 ribosomal RNA genes, and 36 transfer RNA genes. Further phylogenomic analysis showed that F. shweliensis was close to Coffea genus in the family Rubiaceae.
Fosbergia shweliensis (Anth.) Tirveng. and Sastre is a subtropical tree species belonging to the family of Rubiaceae. It is endemic to the Gaoligong Mountains region of Yunnan province, China (Li et al. Citation2013). It has large, yellow, white and fragrant flowers. It is a potential important horticultural economic species (Li et al. Citation2006). It is a potentially critically endangered Chinese tree species (Dao et al. Citation2011). However, there has been no genomic studies on F. shweliensis.
Herein, we reported and characterized the complete F. shweliensis plastid genome. The GenBank accession number is MT180075. One F. shweliensis individual (specimen number: 201908028) was collected from Baoshan, Yunnan Province of China (24°73′13ʺN, 99°18′23ʺE). The specimen is stored at the herbaria, YAF and YCP and the accession number is GYFEP006. DNA was extracted from its fresh leaves using DNA Plantzol Reagent (Invitrogen, Carlsbad, CA, USA).
Paired-end reads were sequenced using Illumina HiSeq system (Illumina, San Diego, CA). In total, about 21.5 million high-quality clean reads were generated with adaptors trimmed. Aligning, assembly, and annotation were conducted by CLC de novo assembler (CLC Bio, Aarhus, Denmark), BLAST, GeSeq (Tillich et al. Citation2017), and GENEIOUS v 11.0.5 (Biomatters Ltd, Auckland, New Zealand). To confirm the phylogenetic position of F. shweliensis, other 13 species of the family Rubiaceae from NCBI were aligned using MAFFT v.7 (Katoh and Standley Citation2013). The auto algorithm in the MAFFT alignment software was used to align the 16 complete genome sequences and the G-INS-i algorithm was used to align the partial complex sequences. The maximum likelihood (ML) bootstrap analysis was conducted using RAxML (Stamatakis Citation2006); bootstrap probability values were calculated from 1000 replicates. Calotropis procera (MH939982) and Hoya carnosa (MN781974) were served as the outgroup.
The complete F. shweliensis plastid genome is a circular DNA molecule with the length of 154,717 bp, contains a large single-copy region (LSC) of 84,747 bp and a small single-copy region (SSC) of 18,230 bp, which were separated by a pair of inverted repeats (IR) regions of 25,870 bp. The overall GC content of the whole genome is 37.6%, and the corresponding values of the LSC, SSC, and IR regions are 35.5%, 31.4%, and 43.2%, respectively. The plastid genome contained 130 genes, including 85 protein-coding genes, 8 ribosomal RNA genes, and 36 transfer RNA genes. Phylogenetic analysis showed that F. shweliensis was close to Coffea genus in the family Rubiaceae (). The determination of the complete plastid genome sequences provided new molecular data to illuminate the family Rubiaceae evolution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dao ZL, Ji YH, Liu T, Liu KM, Li H. 2011. Development of ten polymorphic microsatellite loci for Fosbergia shweliensis (Rubiaceae), a potentially crisis endangered tree. Am J Bot. 98(6):161–163.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol E. 30(4):772–780.
- Li AH, Yang J, He HJ, Yang XY. 2013. Seed desiccation tolerance and germination of a potentially threatened Chinese species, Fosbergia shweliensis. Seed Sci Technol. 41(3):479–482.
- Li H, Dao ZL, Li R. 2006. Reappraisal of Fosbergia shweliensis (Rubiaceae), a species endemic to the Gaoligong mountains, Western Yunnan, China. J Syst E. 44(6):707–711.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22(21):2688–2690.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.