Abstract
In this study, we used next-generation sequencing to obtain the complete mitochondrial genome of Jaydia lineata. This mitochondrial genome, consisting of 16,510 base pairs (bp), contains 13 protein-coding genes, 2 ribosomal RNAs, 22 transfer RNAs and 2 noncoding control regions (control region and origin of light-strand replication) as those found in other vertebrates. Control region, of 848 bp in length, is located between tRNAPro and tRNAPhe. Within the control region, typical conserved domains, such as the termination-associated sequence, central and conserved sequence blocks domains were identified. The overall base composition shows 26.71% of T, 28.46% of C, 27.24% of A and 17.58% of G, with a slight A + T rich feature (53.95%). The complete mitogenome data provides useful genetic markers for the studies on the molecular identification, population genetics, phylogenetic analysis and conservation genetics.
The Indian perch, Jaydia lineata (Temminck & Schlege 1842), is a common species distributed mainly in the Indo-Western Pacific. It is of importance to local ecosystem, and sometimes used as a food fish (Liu et al. Citation2018). Most populations of Indian perch in China are under the threat due to overfishing and habitat destruction fish (Liu et al. Citation2018). At present, only limited mitochondrial sequences have been published for J. lineata. To promote the rational utilization of J. lineata resources, it is necessary to obtain the genetic information of this species. In this study, we presented the complete mitochondrial genome of J. lineata, and some gene markers were further used for the phylogenetic status of this species.
The sample of J. lineata was collected from the coastal water of Qingdao (36.41°N, 120.77°E), China during May 2019. The examined specimen was preserved at Fisheries Ecology and Biodiversity Laboratory in Zhejiang Ocean University under specimen accession NO. ZJOU-04056. The genomic DNA was extracted from dorsal-lateral muscles (30 mg) using Rapid Animal Genomic DNA Isolation Kit (Sangon Biotech Co., Ltd., Shanghai, CN). A genomic library was established and followed by next-generation sequencing. Whole genome resequencing (sequencing depth 50X) was conducted by using Illumina Hiseq4000 platform with the sequencing insertion of 350-bp. Quality check for sequencing data was done by FastQC (Andrews Citation2010) and the filtered clean data were assembled and mapped to complete mitogenome sequence using NOVOPlasty v3.7.2 (Dierckxsens et al. Citation2017). Subsequently, the assembled sequence was annotated using the online Mitochondrial Genome Database of Fish server (Iwasaki et al. Citation2013) and the MITOS Web Server (Bernt et al. Citation2013).
The final sequence has been deposited in GenBank with accession number MT363638. The complete mitochondrial genome of J. lineata (16,510 bp in length) consists of 13 protein-coding genes, 22 tRNA genes, 2 rRNA genes and 2 non-coding control regions (control region and origin of light-strand replication). The arrangement of all genes is identical to those of most vertebrates (Wang et al. Citation2008; Chen Citation2013; Chiang et al. Citation2013). Most of the genes are encoded on the heavy strand (H-strand), except for the eight tRNA genes (-Gln, -Ala, -Asn, -Cys, -Tyr, -Ser, -Glu and -Pro) and one protein-coding gene (ND6). The overall base composition is 26.71% for T, 28.46% for C, 27.24% for A, and 17.58% for G, with a slight A + T-rich feature (53.95%). Except for COI starting with GTG, the remaining 12 protein-coding genes start with ATG. It is important to note that some of the protein-coding genes (3 of 13 genes) are inferred to terminate with an incomplete stop codon (COII, ND4 and Cyt b), with four (ATPase8, ATPase6, ND2 and ND5) sharing TAA and six (COI, COIII, ND1, ND3, ND4L and ND6) using TAG as a stop codon, respectively. These features are common among vertebrate mitochondrial genome, and TAA is supposed to be appeared via posttranscriptional polyadenylation (Ojala et al. Citation1981). The longest one is ND5 gene (1824 bp) among protein-coding genes, whereas the shortest is ATPase 8 gene (168 bp). The non-coding control region (D-loop) is 848 bp in length, and is located between tRNAPro and tRNAPhe. Within D-loop, a termination-associated sequence (TAS), conserved sequence blocks (CSB 1 and CSB 2), and several areas of highly conserved sequence (C, D and F Box) were detected. The two ribosomal RNA genes, 12S rRNA (955 bp) and 16S rRNA (1678 bp), are located between tRNAPhe and tRNALeu.
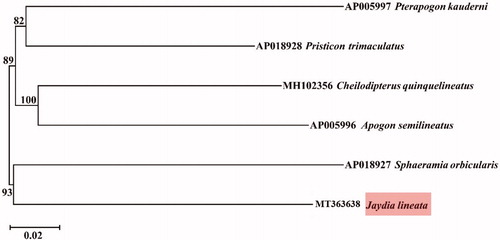
Phylogenetic relationships were constructed using neighbour-joining (NJ) algorithm implemented in MEGA 6 (Tamura et al. Citation2013) among 6 species of family Apogonidae based on 12 H-strand mitochondrial protein-coding genes, 22 tRNA and 2 rRNA genes (). This phylogenetic tree showed that J. lineata has a relatively close relationship with Sphaeramia orbicularis. The information of the mitogenome will be useful for future phylogenetic studies and specimen identification of Apogonidae species.
Acknowledgement
We thank Dr. Linlin Zhao for sample collection and we are grateful to Dr. Zhi Chen for the help of sequence analysis.
Disclosure statement
The authors declare that they do not have any conflict of interest. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The data that support the findings of this study is openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov under accession number MT363638.
Additional information
Funding
References
- Andrews S. 2010. FastQC: A quality control tool for high throughput sequence data. [Accessed 2018 Oct 4]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Chen IS. 2013. The complete mitochondrial genome of Chinese sucker Myxocyprinus asiaticus (Cypriniformes, Catostomidae). Mitochondrial DNA. 24(6):680–682.
- Chiang TY, Chen IS, Lin HD, Chang WB, Ju YM. 2013. Complete mitochondrial genome of Sicyopterus japonicus (Perciformes, Gobiidae). Mitochondrial DNA. 24(3):191–193.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30(11):2531–2540.
- Liu B, Liu Y, Zhu K, Liu L, Jiang L, Gong L, Liu Z, Zhang W, Duan W, Lü Z. 2018. Characterization of complete mitochondrial genome of Jaydia lineata (Perciformes, Apogonidae). Mitochondrial DNA Part B. 3(2):1025–1026.
- Ojala D, Montoya J, Attardi G. 1981. tRNA punctuation model of RNA processing in human mitochondria. Nature. 290(5806):470–474.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729.
- Wang C, Chen Q, Lu G, Xu J, Yang Q, Li S. 2008. Complete mitochondrial genome of the grass carp (Teleostei, Cyprinidae, Gobioninae). Gene. 424(1–2):96–101.