Abstract
Pharyngocirrus Di Domenico, Martínez, Lana & Worsaae, 2014 belongs to the family Saccocirridae Bobretzky, 1872 and comprises 12 species. Of these, Pharynogocirrus uchidai is known as distributed in only East Asia (Korea and Japan). This study reports the first complete mitogenome of the Protodriliformia group as well as Saccocirridae. The mitogenome of P. uchidai was 15,415 bp long and included 13 protein-coding genes, two rRNA genes, 22 tRNA genes, and one control region. Based on the phylogenetic data, it was difficult to assess the phylogenetic position of Saccocirridae because of the low bootstrapping value and lack of mitogenome references of the Protodriliformia group. Nevertheless, this genetic data is important for better understanding of the phylogenetic position of the Protodriliformia group.
Interstitial polychaetes inhabit the spaces between sand grains and sediment particles. As a result of adaptation to interstitial habitats, they have unique characteristics such as small body size, adhesive organs, weak segmentation, spionid-like primary palps, few or no parapodia, few or no chaetae, few or no appendages, no circular musculature etc. (Swedmark Citation1964; Orrhage Citation1974; Westheide Citation1987; Worsaae and Kristensen Citation2005; Westheide Citation2008). Eight families, including Diurodrilidae Kristensen & Niilonen, 1982, Nerillidae Levinsen, 1883, Parergodrilidae Reisinger, 1925, Polygordiidae Czerniavsky, 1881, Protodrilidae Hatschek, 1888, Protodriloididae Purschke & Jouin, 1988, Psammodrilidae Swedmark, 1952, and Saccocirridae Bobretzky, 1872 are considered true interstitial polychaetes, as all these species are meiofaunal (Hermans Citation1969; Worsaae and Kristensen Citation2005; Westheide Citation2008). Saccocirridae is placed in the Protodriliformia group within the subclass Errantia, along with Protodrilidae, Protodriloididae, and Polygordiidae, based on molecular phylogenetic data (Andrade et al. Citation2015; Struck et al. Citation2015; Weigert and Bleidorn Citation2016). Saccocirridae is further classified into two genera: Saccocirrus Bobretzky, 1872 and Pharyngocirrus Di Domenico, Martínez, Lana & Worsaae, 2014. A total of 12 species have been reported in the genus Pharyngocirrus. Particularly, Pharyngocirrus uchidai (Sasaki Citation1981) is distributed in Oshoro Bay in Japan and the eastern coast of Korea (Sasaki Citation1981; Park et al. Citation2019).
Pharyngocirrus uchidai specimen was collected from the sandy environment of Oeongchi beach, Sokcho, Republic of Korea (38°11′00″ N, 128°36′34″ E), and was deposited at the National Institute of Biological Resources (Registration number: NIBRIV0000863976). Genomic DNA was extracted from the whole body using DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). REPLI-g Mitochondrial DNA Kit (Qiagen) was used for mitochondrial DNA amplification and mitochondrial genome sequencing was performed using the HiSeq 2000 sequencing system (Illumina, San Diego, CA, USA).
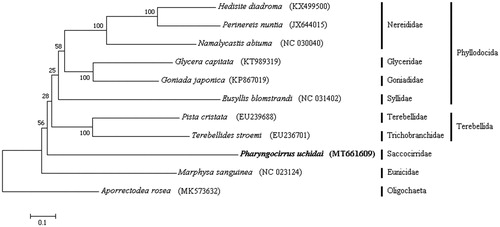
The complete mitogenome of P. uchidai (Accession number MT661609) was 15,415 bp long and consisted of 13 protein-coding genes, two rRNA genes, 22 tRNA genes, and one control region. The overall nucleotide composition was 22.2% A, 26.0% C, 18.5% G, and 33.3% T, with a high A + T content (55.5%). Moreover, the annotated P. uchidai sequence was compared with those of other annelids, specifically the 10 species belonging to eight families registered in the GenBank. Phylogenetic analysis was performed using the maximum likelihood method and the GTR + G+I model with a bootstrap of 1000 replicates.
Based on the obtained phylogenetic data alone, it is difficult to examine the phylogenetic position of Saccocirridae because of the low bootstrapping value and lack of mitogenome reference of the Protodriliformia group. Nevertheless, this is the first complete mitogenome description among the Protodriliformia group. Therefore, this study may provide important clues to elucidate the phylogenetic position of these particular interstitial polychaetes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in Mendeley Data at http://dx.doi.org/10.17632/8bzn6vy7th.1
Additional information
Funding
References
- Andrade SC, Novo M, Kawauchi GY, Worsaae K, Pleijel F, Giribet G, Rouse GW. 2015. Articulating “archiannelids”: phylogenomics and annelid relationships, with emphasis on meiofaunal Taxa. Mol Biol Evol. 32(11):2860–2875.
- Hermans CO. 1969. The systematic position of the Archiannelida. Syst Zool. 18(1):85–102.
- Orrhage L. 1974. Über die Anatomie, Histologie und Verwandtschaft der Apistobranchidae (Polychaeta, Sedentaria) nebst Bemerkungen über die systematische Stellung der Archianneliden. Z Morph Tiere. 79(1):1–45.
- Park J, Kajihara H, Jung J. 2019. First record of the interstitial Annelid Pharyngocirrus uchidai (Annelida: Saccocirridae) from Korea, confirmed by topotypic DNA barcoding data from Japan. Animal Syst Evol Diversity. 35(1):33–36.
- Sasaki S. 1981. A new species of the genus Saccocirrus (Archiannelida) from Hokkaido, northern Japan. Annotationes Zoologicae Japonenses. 54(4):259–266.
- Struck TH, Golombek A, Weigert A, Franke FA, Westheide W, Purschke G, Bleidorn C, Halanych KM. 2015. The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr Biol. 25(15):1993–1999.
- Swedmark B. 1964. Interstitial fauna of marine sand. Biol Rev. 39(1):1–42.
- Weigert A, Bleidorn C. 2016. Current status of annelid phylogeny. Org Divers Evol. 16(2):345–362.
- Westheide W. 1987. Progenesis as a principle in meiofauna evolution. J Nat Hist. 21(4):843–854.
- Westheide W. 2008. Polychaetes: interstitial families. London: Synopsis of the British Fauna; p. 152.
- Worsaae K, Kristensen RM. 2005. Evolution of interstitial Polychaeta (Annelida). Hydrobiologia. 179:319–340.