Abstract
In this study, we sequenced the complete mitochondrial genome of Eutrichosiphum pasaniae through Illumina platform. The circular mitogenome is 16,500 bp in length and composed of 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), 2 ribosomal RNA genes (rRNAs), a large control region and a special repeat region. The nucleotide composition of whole mitogenome is strongly AT-biased (85.5%). All PCGs start with ATN and end with TAA except for cox1 which terminates with an incomplete stop codon T. All tRNAs have a typical clover-leaf secondary structure except for trnS (AGN). The lengths of rrnL, rrnS and control region are 1276, 774 and 996 bp, respectively. The repeat region with a length of 909 bp is located between trnE and trnF and consists of 4.1 repeat units. The phylogenetic tree supports the sister relationship of Eutrichosiphum pasaniae and Greenidea psidii.
Keywords:
The aphid species Eutrichosiphum pasaniae (Okajima, 1908) (Hemiptera: Aphididae: Greenideinae) distributes in eastern and southeastern Asia and mainly feeds on leaves and young shoots of the plants of Lithocarpus and Castanopsis (Blackman and Eastop Citation2020). In the present study, we sequenced the complete mitochondrial genome of E. pasaniae through the Illumina platform. The E. pasaniae samples were collected from Castanopsis uraiana in Daren Township, Taiwan, China (22.3750°N, 120.8592°E) and deposited in the National Zoological Museum of China, Institute of Zoology, Chinese Academy of Science, Beijing, China (NZMC no. 39256).
The circular mitochondrial genome of E. pasaniae is 16,500 bp long (GenBank accession number MT883997) and includes 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), 2 ribosomal RNA genes (rRNAs), a control region and a special repeat region between trnE and trnF. The gene order is identical to the inferred ancestral arrangement of insects (Clary and Wolstenholme Citation1985). The majority strand contains 9 PCGs and 14 tRNAs, while the remaining genes are located on the minority strand. The overall nucleotide composition of E. pasaniae mitogenome is 46.6% A, 38.9% T, 5.5% G and 9.1% C, which is strongly AT-biased (85.5%). In the whole mitogenome, there are 21 intergenic spacers ranging from 1 to 51 bp and 8 gene overlapping regions ranging from 1 to 20 bp.
Thirteen PCGs are initiated by the standard ATN and ended with TAA expect for cox1, which uses an incomplete stop codon T. The tRNA genes range from 62 to 73 bp in length and are predicted to possess a classical clover-leaf secondary structure expect for trnS (AGN), the dihydrouridine (DHU) arm of which forms a simple loop. The lengths of rrnL and rrnS genes are 1276 and 774 bp, with an A + T content of 85.3 and 83.8%, respectively. The control region is 996 bp long and located between rrnS and trnI, with an A + T content of 92.2%. A large repeat region is present between trnE and trnF, which is unique and species-specific in aphids (Wang et al. Citation2015). The repeat region is 909 bp long with an A + T content of 87.6% and consists of a 222-bp repeat unit which is repeated 4.1 times.
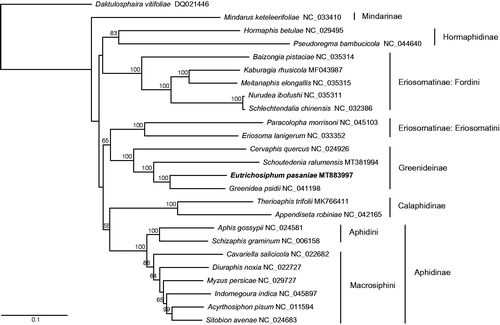
To perform the phylogenetic analysis, we used the whole mitogenome sequences of E. pasaniae and 24 other aphid species. The maximum-likelihood phylogenetic tree was constructed with RAxML v8.2.10 (Stamatakis Citation2014). The subfamily Greenideinae was monophyletic and E. pasaniae was placed as a sister to Greenidea psidii ().
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.zkh189388.
Additional information
Funding
References
- Blackman RL, Eastop VF. 2020. Aphids on the world’s plants; [accessed 2020 Sep 4]. http://www.aphidsonworldsplants.info/.
- Clary DO, Wolstenholme DR. 1985. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 22(3):252–271.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Wang Y, Chen J, Jiang LY, Qiao GX. 2015. Hemipteran mitochondrial genomes: features, structures and implications for phylogeny. Int J Mol Sci. 16(6):12382–12404.