Abstract
Colobopsis ants are unique ants known for their phragmotic behavior. We have completed the mitochondrial genome of Colobopsis nipponica (Wheeler, W.M., 1928) as the first mitochondrial genome of the genus. The mitogenome is 17,431 bp long and 19.4% in GC ratio, which is the third longest mitochondrial genome in subfamily Formicinae. It contains 13 protein-coding genes, 2 rRNA genes, 22 tRNA genes and a large 1534 bp long control region. Both gene order and phylogenetic analysis agree with the recent elevation of Colobopsis from subgenus to genus.
Gate-keeper ants of genus Colobopsis are well known for their phragmotic behavior of acting as ‘living doors’. They are mainly distributed in the Indo-Australian regions, but a handful of species also inhabit the Holarctic and northern parts of the Neotropical regions (Janicki et al. Citation2016). Most species of this genus have specialized major workers that have sharp truncated heads. Within their arboreal nests, the major workers use their heads to plug their nest entrances, blocking intruders but allowing nestmates to pass (Ward et al. Citation2016). Until recently, Colobopsis was considered a subgenus of the mega-diverse carpenter ant genus Camponotus, but was elevated to a proper genus as phylogenomic analysis revealed Colobopsis was sister to all other members of tribe Camponotini, while other Camponotus nested inside the tribe (Ward et al. Citation2016). As the first mitochondrial genome of this fascinating genus, we have completed the mitogenome of Colobopsis nipponica, a species native to east Asia.
The ants were collected from a colony found in a branch of a Camellia japonica tree, in a coastal forest of Geoje Island, Republic of Korea (34°51′59.6″N, 128°44′22.0″E). Total DNA was extracted from worker ants using DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany). Sequencing library was constructed using Illumina TruSeq Nano DNA Library Preparation Kit (Illumina, San Diego, CA) following the manufacturer’s recommendations with around 350-bp DNA fragments. 4.46 Gbp raw sequences obtained from Illumina HiSeqX at Macrogen Inc., Korea, were filtered by Trimmomatic v0.33 (Bolger et al. Citation2014), de novo assembled, and confirmed by Velvet v1.2.10 (Zerbino and Birney Citation2008), SOAPGapCloser 1.12 (Zhao et al. Citation2011), BWA v0.7.17 (Li et al. Citation2009), and SAMtools v1.9 (Song and Liang Citation2013) under the environment of Genome Information System (GeIS; http://geis.infoboss.co.kr). Geneious R11 v11.1.5 (Biomatters Ltd, Auckland, New Zealand) was used to annotate the mitogenome based on alignments with other ant mitogenomes and MITOS (Bernt et al. Citation2013) was used to double check annotated genes. DNA sample and specimen (95% ethanol) were deposited in InfoBoss Cyber Herbarium (IN; J. Park, KFDS00186).
The mitochondrial genome of C. nipponica (GenBank accession: MW067133) is 17,431 bp long and 19.4% in GC ratio, which is the third longest mitochondrial genome in subfamily Formicinae, only shorter than that of Acropyga kinomurai (NC_046423; 18,287 bp) and Aphomomyrmex afer (MK861054; 17,844 bp). Thirty-seven genes including 13 protein-coding genes (PCGs), 2 rRNAs, and 22 tRNAs are identified in an order that is shared by most members of the subfamily and is considered the ancestral gene order of the subfamily (Vieira and Prosdocimi Citation2019). In tribe Camponotini, Polyrhachis dives also shares the same ancestral order (Liu et al. Citation2017) but Camponotus spp. does not as a transposition of trnI is always present in Camponotus species (Kim et al. Citation2016; Park et al. Citation2019). As C. nipponica retains the ancestral gene order that is shared with P. dives but not with Camponotus spp., we can suggest that the mitochondrial gene order also supports the separation of Colobopsis from true Camponotus species. Large size of C. nipponica mitogenome could be attributed to its long control region of 1,534 bp, in which multiple sets of tandem repeats are found.
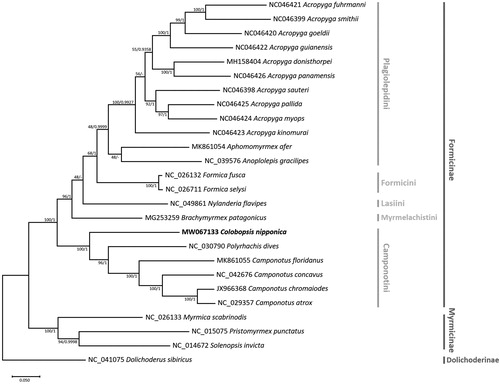
Phylogenetic analysis was conducted using all available 22 formicine mitogenomes containing all 13 PCGs including C. nipponica and 4 outgroup species. Sequences of 13 PCGs from 26 mitogenomes were extracted and codon-based alignment was conducted for each PCG using MAFFT provided on the Translator X web server with default settings (Abascal et al. Citation2010). Concatenated alignment of 13 PCGs was subjected to construct a maximum-likelihood (ML) tree using MEGA X (Kumar et al. Citation2018) and a Bayesian Inference (BI) tree using MrBayes v3.2.7a (Ronquist et al. Citation2012). A heuristic search was used with nearest-neighbor interchange branch swapping, Tamura-Nei model, and uniform rates among sites to construct ML tree. In both phylogenetic trees, C. nipponica was sister to all other Camponotini species (), congruent to the previous phylogenomic tree drawn with 959 ultra-conserved element (UCE) loci (Blaimer et al. Citation2015). Tribe Camponotini was sister to the rest of subfamily Formicinae (), which is incongruent with the UCE phylogenomic tree but is a known phenomenon of mitochondrial gene-based trees of Formicinae (Chen et al. Citation2013). Topology of remaining formicine tribes was congruent to the UCE phylogenomic tree in the ML tree but was not in the BI tree as tribes Lasiini and Myrmelachistini grouped in the latter tree. This could be improved when more mitogenomes are available covering other unexplored genera since only a single mitogenome is available for both tribes. The mitogenome of C. nipponica will be invaluable in further understanding of mitochondrial evolution in ants.
Figure 1. Maximum-likelihood (bootstrap repeat: 10,000) and Bayesian Inference (1,100,000 generations) phylogenetic trees of all available 22 Formicinae ant mitochondrial genomes: Colobopsis nipponica (MW067133, this study), Camponotus atrox (NC_029357), Camponotus chromaiodes (JX966368), Camponotus concavus (NC_042676), Camponotus floridanus (MK861055), Polyrhachis dives (NC_030790), Acropyga donisthorpei (MH158404), Acropyga fuhrmanni (NC046421), Acropyga goeldii (NC046420), Acropyga guianensis (NC046422), Acropyga kinomurai (NC046423), Acropyga myops (NC046424), Acropyga pallida (NC046425), Acropyga panamensis (NC046426), Acropyga sauteri (NC046398), Acropyga smithii (NC046399), Anoplolepis gracilipes (NC_039576), Aphomomyrmex afer (MK861054), Formica fusca (NC_026132), Formica selysi (NC_026711), Nylanderia flavipes (NC_049861), Brachymyrmex patagonicus (MG253259), and four outgroup species: Myrmica scabrinodis (NC_026133), Solenopsis invicta (NC_014672), Pristomyrmex punctatus (NC_015075), and Dolichoderus sibiricus (NC_041075). Phylogenetic tree was drawn based on maximum-likelihood tree. The numbers above branches indicate bootstrap support values of maximum likelihood tree and posterior probability of Bayesian Inference tree, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Mitochondrial genome sequence can be accessed via accession number MW067133 in GenBank of NCBI at https://www.ncbi.nlm.nih.gov. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA667962, SAMN16392962, and SRR12791361, respectively.
Additional information
Funding
References
- Abascal F, Zardoya R, Telford MJ. 2010. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 38(Web Server issue):W7–W13.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Blaimer BB, Brady SG, Schultz TR, Lloyd MW, Fisher BL, Ward PS. 2015. Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: a case study of formicine ants. BMC Evol Biol. 15(1):271.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Chen Z, Zhou SY, Ye D, Chen Y, Lu C. 2013. Molecular phylogeny of the ant subfamily Formicinae (Hymenoptera, Formicidae) from China based on mitochondrial genes. Sociobiology. 60(2):135–144.
- Janicki J, Narula N, Ziegler M, Guénard B, Economo EP. 2016. Visualizing and interacting with large-volume biodiversity data using client–server web-mapping applications: the design and implementation of antmaps.org. Ecol Inf. 32:185–193.
- Kim MJ, Hong EJ, Kim I. 2016. Complete mitochondrial genome of Camponotus atrox (Hymenoptera: Formicidae): a new tRNA arrangement in Hymenoptera. Genome. 59(1):59–74.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25(16):2078–2079.
- Liu J-H, Jia P-F, Fu J-Q, Dan W-L, Yang L-Y, Wang Q-M, Li Z-N. 2017. Characterization of mitochondrial genome and phylogenetic implications for Chinese black ant, Polyrhachis dives (Hymenoptera: Formicidae). Mitochondrial DNA Part B. 2(2):679–680.
- Park J, Kwon W, Park J. 2019. The complete mitochondrial genome of Camponotus concavus Kim & Kim, 1994 (Hymenoptera: Formicidae). Mitochondrial DNA Part B. 4(1):1243–1244.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Song N, Liang A-P. 2013. A preliminary molecular phylogeny of planthoppers (Hemiptera: Fulgoroidea) based on nuclear and mitochondrial DNA sequences. PLoS One. 8(3):e58400.
- Vieira GA, Prosdocimi F. 2019. Accessible molecular phylogenomics at no cost: obtaining 14 new mitogenomes for the ant subfamily Pseudomyrmecinae from public data. PeerJ. 7:e6271
- Ward PS, Blaimer BB, Fisher BL. 2016. A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa. 4072(3):343–357.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18(5):821–829.
- Zhao QY, Wang Y, Kong YM, Luo D, Li X, Hao P. 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinf. 12(Suppl 14):S2.
