Abstract
In this study, the complete mitochondrial genome of Cottiusculus nihonkaiensis was presented, and we also discussed its mitochondrial characteristics. The full length of the mitochondrial genome was 16,612 bp, containing 13 protein-coding genes (PCGs), two ribosomal RNAs (12S and 16S), 22 transfer RNA genes (tRNA), one non-coding control region (CR) and one origin of replication on the light-strand. Overall base composition of the complete mitochondrial DNA was 26.4% A, 17.4% G, 31.5% C, 24.7% T. The phylogenetic tree suggested that C. nihonkaiensis shared the most recent common ancestor with Gymnocanthus herzensteini, Gymnocanthus intermedius and Gymnocanthus tricuspis.
Cottiusculus nihonkaiensis in the family Cottidae (Scorpaeniformes) is distributed the Sea of Japan, Sea of Okhotsk and Pacific coast of Hokkaido (Kai and Nakabo Citation2009). It is a temperate marine fish, inhabiting in the bottom sea area (Kai and Yamanaka Citation2019). In this study, we described the complete mitochondrial genome of C. nihonkaiensis and analyzed the phylogenetic relationship of Scorpaeniformes, to gain its molecular information and thus contribute to facilitate future studies on population genetic structure and phylogenetic relationships.
The sample of C. nihonkaiensis was collected from the East China Sea (25°54′15′N, 117°05′24′E) and stored in 95% alcohol, then kept in the laboratory of Zhejiang Ocean University with accession number 20170927SJL25. Total genomic DNA was extracted using a phenol-chloroform extraction protocol (Kchl et al. Citation2005). Subsequently, based on the existing complete mitochondrial gene of Gymnocanthus herzensteini (KX148474), 18 pairs of primers were designed, the samples were amplified by PCR, and then sequenced using Sanger sequencing technology. NOVOPlasty software was used to assemble the mitogenomes, the mistake parameter was set by default (Dierckxsens et al. Citation2017). The boundaries of ribosomal RNA genes were identified by a BLAST search (http://blast.ncbi.nlm.nih.gov). The locations of the transfer RNAs and protein-coding genes were identified using the program tRNAscan-SE version 2.0 (http://trna.ucsc.edu/tRNAscan-SE/) (Li et al. Citation2013) and MITOS WebServer (http://mitos2.bioinf.uni-leipzig.de/index.py), respectively. The one origin of replication on the light-strand and control region were determined by the proposed secondary structures and sequence homology. The whole mitochondrial genome of C. nihonkaiensis was a closed double-stranded circular molecule consisting of 16,612 bp (GenBank accession number: MK224511), which was very similar to other typical vertebrate mitochondria (Miya et al. Citation2001; Zhu et al. Citation2018a, Citation2018b). The complete mitochondrial genome contains 13 protein-coding genes (PCGs), 22 transfer RNAs (tRNA) genes, two ribosomal RNA genes (12S rRNA and 16S rRNA), a putative control region (CR) and one origin of replication on the light-strand (OL). The overall base composition was 26.4% A, 17.4% G, 31.5% C, 24.7% T, respectively, with a slight AT bias (51.1%). C. nihonkaiensis mitochondrial genes were mostly encoded on the heavy strand, and only ND6 and eight tRNA (Gln, Ala, Asn, Cys, Tyr, Ser, Glu and Pro) genes on the light strand coding. The start codons of the 13 PCGs encoding genes were ATG except for COI which was GTG, which is quite common in vertebrate mtDNA (He et al. Citation2015). Most of the stop codons were TAA or T––, the stop codon of ND2 was CTA and the gene with TTA as the stop codon was COIII. Most of the tRNAs could form a common cloverleaf secondary structure, except tRNASer (GCT) gene without DHU stem (Han et al. Citation2016). The lengths of the two rRNA genes were 947 bp (12S rRNA) and 1,692 bp (16S rRNA), respectively, which located between the tRNAPhe and tRNALeu (TAA) and separated by the tRNAVal gene. The length of the control region was 858 bp, located between tRNAphe and tRNAPro.
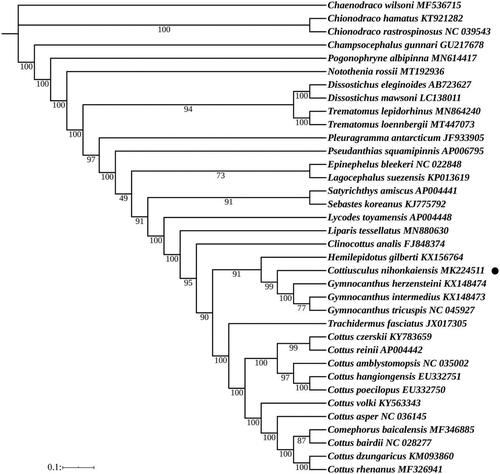
In order to obtain the position and kinship of the C. nihonkaiensis within Scorpaeniformes, we constructed phylogenetic trees of Scorpaeniformes based on maximum likelihood (ML) method (). According to the Akaike Information Criteria (AIC), the most suitable nucleotide sequence model was selected through MrModeltest 2.3 (Bozdogan Citation1987), and finally the most suitable model was GTR + I + G. The ML phylogenetic tree based on 13 PCGs of 36 species using the software RAxML 8.0 (Alexandros Citation2014). The results showed that C. nihonkaiensis shared the most recent common ancestor with Gymnocanthus herzensteini, Gymnocanthus intermedius and Gymnocanthus tricuspis.
Figure 1. Phylogenetic analysis based on the nucleotide sequences of the 13 PCGs in the mitogenome. Maximum Likelihood analyses (bootstrap support with 1000 replications) are shown next to nodes. The number after the species name was the GenBank accession number. The genome sequence in this study was labeled with a black dot.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in “NCBI” athttps://www.ncbi.nlm.nih.gov/nuccore/MK224511. GenBank accession number: MK224511.1.
Additional information
Funding
References
- Alexandros S. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. (9):1312–1313.
- Bozdogan H. 1987. Model selection and Akaike's Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika. 52 (3):345–370.
- Dierckxsens N, Patrick M, Guillaume S. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45 (4):e18.
- Han H, Wang N, Xu L, Gao S, Liu A. 2016. The complete mitochondrial genome of Calliptamus abbreviatus Ikovnnikov (Orthoptera: acridoidea). Mitochondrial DNA Part B. 1 (1):770–771.
- He SL, Zou Y, Zhang LF, Ma WQ, Zhang XY, Yue BS. 2015. The complete mitochondrial genome of the beet webworm, Spoladea recurvalis (Lepidoptera: Crambidae) and its phylogenetic implications. PLOS One. 10(6):e0129355.
- Kai Y, Nakabo T. 2009. Taxonomic review of the genus Cottiusculus (Cottoidei: Cottidae) with description of a new species from the Sea of Japan. Ichthyol Res. 56 (3):213–226.
- Kai Y, Yamanaka T. 2019. Tsugaru Strait hybrid zone between two Japanese marine sculpins (genus Cottiusculus). Mar Biodiv. 49(1):501–504.
- Kchl S, Niedersttter H, Parson W. 2005. DNA extraction and quantitation of forensic samples using the phenol-chloroform method and real-time PCR. Methods Mol Biol. 297(13).
- Li X, Yang J, Wang JH, Ren QL, Xia LI, Huang Y. 2013. Methods and software tools for mitochondrial genome assembly and annotation. Chin J Appl Entomol.
- Miya M, Kawaguchi A, Nishida M. 2001. Mitogenomic exploration of higher teleostean phylogenies: a case study for moderate-scale evolutionary genomics with 38 newly determined complete mitochondrial DNA sequences. Mol Biol Evol. 18 (11):1993–2009.
- Zhu K, Gong L, Jiang L, Liu L, Lü Z, Liu B-j. 2018a. Phylogenetic analysis of the complete mitochondrial genome of Anguilla japonica (Anguilliformes, Anguillidae). Mitochondrial DNA Part B. 3 (2):536–537.
- Zhu K, Gong L, Lü Z, Liu L, Jiang L, Liu B. 2018b. The complete mitochondrial genome of Chaetodon octofasciatus (Perciformes: Chaetodontidae) and phylogenetic studies of Percoidea. Mitochondrial DNA Part B. 3(2):531–532.
