Abstract
Crataegus hupehensis Sarg. is well-known for its medicinal and nutritive value. In this study, the complete chloroplast genome sequence of C. hupehensis was determined by using Illumina high-throughput sequencing approach. The complete chloroplast genome is 159,766 bp with 36.6% GC content. It contained a pair of inverted repeat regions of 26,385 bp, a large single-copy region of 87,852 bp, and a small single-copy region of 19,144 bp. It contained 112 distinct genes, including 78 protein-coding genes, 4 ribosomal RNA genes, and 30 transfer RNA genes. Phylogenetic analysis based on chloroplast genomes indicated that C. hupehensisis was closely related to C. kansuensis and C. marshallii in the subfamily Maloideae. This complete chloroplast genome will provide valuable insight into evolution, molecular breeding, and phylogenetic analysis of Crataegus species.
Crataegus hupehensis Sarg. (family: Rosaceae) is a medicinal and edible plant belonging to Crataegus species which are widely distributed in the temperate regions of the northern hemisphere in Europe, Asia, and North America (Phipps et al. Citation1990; Du et al. Citation2019). The genus Crataegus L., commonly known as hawthorn, are one of the most economically important plant groups in China, owing to their nutrient-rich fruit and significant medicinal values (Xu et al. Citation2016). Hawthorns contain bioactive components, such as flavonoids, phenols and oligomeric procyanidins, that are widely used in traditional Chinese medicine (Rigelsky and Sweet Citation2002; Dahmer and Scott Citation2010).
China is the main center of Crataegus cultivation, and the place of origin of both cultivated and some wild Crataegus species (Guo and Jiao Citation1995). A total of 18 species and six varieties of Crataegus are widely distributed across China (Zhao and Feng Citation1996). Among these taxa, C. hupehensis, C. pinnatifida var. major, C. bretschneideri, and C. scabrifolia are cultivated (Du et al. Citation2019; Ma et al. Citation2019). However, the interspecies relations and origins, and evolution of the four cultivated Crataegus species remain unknown. Furthermore, genomic resources of Crataegus species are limited. Therefore, we reported the complete chloroplast genome of C. hupehensis based on Illumina sequencing data (GenBank accession number MW201730), which would be helpful for molecular breeding and phylogenetic analysis.
A single individual of C. hupehensis was collected from the Hawthorn Germplasm Repository of Beijing Academy of Forestry and Pomology Sciences (39°97′N, 116°23′E) in Beijing, China. The voucher specimen (accession number: BJLGY-2020-SZ001) was deposited at the Herbarium of Beijing Academy of Forestry and Pomology Sciences (BAFPS-H, http://www.lgs.baafs.net.cn/, Yuanyong Qi, [email protected]). DNA extraction was performed according to a modified CTAB protocol (Li et al. Citation2013) and paired-end libraries were prepared with the NEBNext Ultra DNA Library Prep Kit. High-throughput sequencing was carried out using the HiSeq Xten PE150 System (Illumina, San Diego, CA, USA) with150bp pair-end reads. In all, 1.17 G raw reads were obtained, and after the quality-trimmed using the software CLC Genomics Workbench v7.5 (CLC bio, Aarhus, Denmark), 0.97 G qualified reads were assembled using SPAdes 3.6.1 (Kmer = 95) (Bankevich et al. Citation2012) to contigs. The contigs of chloroplast genome were selected with the BLAST program (Altschul et al. Citation1990), taking the closely related species C.marshallii (MK920293) as a reference, and the selected contigs were assembled using Sequencher 4.10 (https://www.genecodes.com/) software tools. Annotation was performed using the Plann (Huang and Cronk Citation2015), then a physical map of the chloroplast genome generated by Genome Vx (Conant and Wolfe Citation2008).
The size of C. hupehensis chloroplast genome was 159,766 bp with 36.6% GC content. It contained a large single-copy (LSC) region of 87,852 bp, a small single-copy (SSC) region of 19,144 bp, and two inverted repeat (IR) regions of 26,385 bp. The chloroplast DNA of C. hupehensis comprised a total of 112 unique genes, including 78 protein-coding genes, 30 tRNA genes, and 4 rRNA genes. In these genes, 18 genes were duplicated in the IR regions, 15 genes harbored a single intron, and 2 (ycf3, clpP) contained double introns.
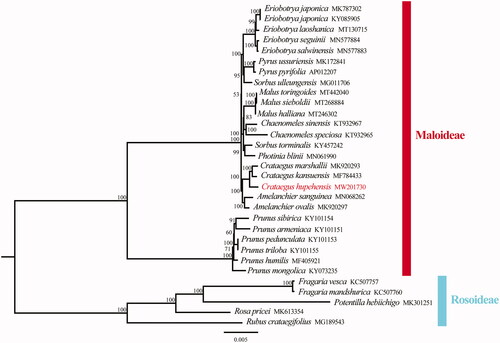
To clarify the phylogenetic position of C. hupehensis, total 31 complete chloroplast genomes were obtained from Genbank and the sister group Rosoideae was taken as an outgroup. All chloroplast genome sequences were aligned using MAFFT (Katoh et al. Citation2019) and phylogenetic analysis was conducted based on maximum-likelihood (ML) analyses using IQ-TREE (1.6.12) with 1000 bootstrap replicates (Nguyen et al. Citation2015). The phylogenetic analysis showed that C. hupehensis was closely related to C. kansuensis and C. marshallii in the subfamily Maloideae (). This complete chloroplast genome can be used for future studies on genetic engineering, population and phylogeny of family Rosaceae.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW201730. The associated BioProject, SRA, and Bio-Sample numbers are PRJN A660005, SUB8681476, and SAMN16998579 respectively.
Additional information
Funding
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403–410.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Conant GC, Wolfe KH. 2008. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 24(6):861–862.
- Dahmer S, Scott E. 2010. Health effects of hawthorn (complementary and alternative medicine). Am Fam Physician. 81(4):465–468.
- Du X, Zhang X, Bu XD, Zhang TC, Lao YC, Dong WX. 2019. Molecular analysis of evolution and origins of cultivated hawthorn (Crataegus spp.) and related species in China. Front Plant Sci. 10:443.
- Guo TJ, Jiao PJ. 1995. Hawthorn (Crataegus) resources in China. HortSci. 30(6):1132–1134.
- Huang DI, Cronk Q. 2015. Plann: a command-line application for annotating plastome sequences. Appl Plant Sci. 3(8):1500026.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Li JL, Wang S, Yu J, Wang L, Zhou SL. 2013. A modified CTAB protocol for plant DNA extraction. Chin Bull Bot. 48(1):72–78.
- Ma SLY, Dong WX, Lyu T, Lyu YM. 2019. An RNA sequencing transcriptome analysis and development of EST-SSR markers in Chinese hawthorn through Illumina sequencing. Forests. 10(2):82.
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Phipps JB, Robertson KR, Smith PG, Rohrer JR. 1990. A checklist of the subfamily Maloideae (Rosaceae). Can J Bot. 68(10):2209–2269.
- Rigelsky JM, Sweet BV. 2002. Hawthorn: pharmacology and therapeutic uses. Am J Health Syst Pharm. 59(5):417–422.
- Xu J, Zhao Y, Zhang X, Zhang L, Hou Y, Dong W. 2016. Transcriptome analysis and ultrastructure observation reveal that hawthorn fruit softening is due to cellulose/hemicellulose degradation. Front Plant Sci. 7:1524.
- Zhao HC, Feng BT. 1996. China fruit-plant monograph of hawthorn (Crataegus) Flora. Beijing: Zhongguo Linye Press.