Abstract
The complete mitochondrial genome of the Metaphycus eriococci (Timberlake, 1916) (Hymenoptera: Encyrtidae) was obtained via next-generation sequencing. This mitochondrial genome is 15,749 bp in length with 37 classical eukaryotic mitochondrial genes and an A + T-rich region. All the 13 PCGs begin with typical ATN codons. Among them, 12 PCG genes terminate with TAA, only one with TAG. All of the 22 tRNA genes, ranging from 58 to 72 bp with typical cloverleaf structure except for trnS1 and trnE, whose dihydrouridine arm forms a simple loop. A dramatic gene rearrangement with a large inversion of six protein-coding genes (nad3-cox3-atp6-atp8-cox2-cox1) also found in M. eriococci. Phylogenetic analysis highly supported the monophyly of Pteromalidae, Eupelmidae, and Encyrtidae are sister groups. Within Encyrtidae, Metaphycus eriococci and Aenasius arizonensis are close to each other.
Metaphycus eriococci (Timberlake) (Hymenoptera: Encyrtidae) is a primary parasitoid of eriococcids and may play an important role in biological control of eriococcid pest species (Ghahari Citation2010; Wang et al. Citation2013). The M. eriococci in this study was reared from Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae) which have caused esthetic and economic damage to crape myrtles and poses potential threats to other horticultural crops in the USA (Xie et al. Citation2020). Here, we present the complete mitochondrial genome of M. eriococci.
Specimen of M. eriococci was reared from A. lagerstroemiae collected at Beijing (40.0059°N, 116.3837°E) in July 2020. Voucher specimens of this study were deposited in the Institute of Zoology, Chinese Academy of Sciences (IZCAS) (Voucher number: ZJ20003). The total mitochondrial genome of M. eriococci was obtained through next-generation sequencing. The extracted DNA mixture were applied for library constructing by the usage of Illumina TruSeq@ DNA PCR-Free HT Kit, and sequenced by the platform of llumina HiSeq sequencer (150 bp pared-end). The mitochondrial genome of M. eriococci was assembled based on Illumina short reads with MitoZ v2.3 (Meng et al. Citation2019). The whole mitochondrial genome annotation was annotated by Mitos WebServer (http://mitos2.bioinf.uni-leipzig.de/index.py) under the invertebrate mitochondrial code (Bernt et al. Citation2013). Transfer RNA (tRNA) genes were confirmed by online ARWEN (http://130.235.46.10/ARWEN/) (Laslett and Canback Citation2008). The GenBank accession number of M. eriococci is MW255970.
The complete mitogenome sequence of M. eriococci was 15,749 bp in length with A + T content of 84.2%. It consists of 13 protein-coding genes (PCGs), 22 transfer RNAs (tRNAs), 2 ribosomal RNAs (rRNAs), and a putative control region (CR). All 13 PCGs were initiated by typical ATN codons (six ATT, five ATG, one ATA, and one ATC). Twelve genes use TAA as terminal stop and one gene stop with TAG. All of the 22 tRNA genes, ranging from 58 to 72 bp, have a typical cloverleaf structure except for trnS1 and trnE, whose dihydrouridine (DHU) arm forms a simple loop. The absence of the DHU arm in trnS1 was found in the mitochondrial genomes existed in most insects (Wolstenholme Citation1992). The control region was 688 bp long and 90.1% A + T content. The rrnL and rrnS genes are 1297 and 781 bp, A + T content of them both are 87.6%.
The inversion of six PCGs (including nad3, cox3, atp6, atp8, cox2, and cox1) also been found in M. eriococci which was consisted with other chalcidoids (Oliveira et al. Citation2008). In addition to the previous studies reported that atp8-cox2, nad3-nad5 were gene rearrangement “hot spot” in Hymenoptera (Dowton and Austin Citation1999; Dowton et al. Citation2003), the inversion of six PCGs and nad2-nad3 became the new gene rearrangement ‘hot spot’ in Chalcidoidea.
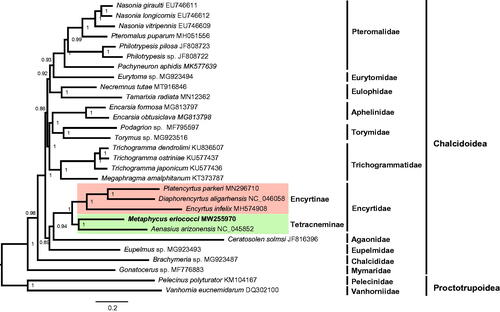
The mitogenomic sequences of 29 chalcidoid species were used to reconstruct the phylogeny of Chalcidoidea. Two species from superfamily Proctotrupoidea (Vanhornia eucnemidarum and Pelecinus polyturator) were chosen as outgroup. Phylogenetic analyses based on 13 PCGs even there were incomplete PCGs in some species using MrBayes (Ronquist et al. Citation2012). The nodes of Bayesian inference phylogeny tree with high support value (). Generally, Mymaridae was always at the basal position within Chalcidoidea (Sharkey et al. Citation2012; Heraty et al. Citation2013). The monophyly of Encyrtidae was strongly supported, shown a sister relationship with Eupelmidae (Xiong et al. Citation2019). Within Encyrtidae, the monophyly of subfamily Encyrtinae and Tetracneminae were also strongly supported.
Disclosure statement
No potential conflict of interest was reported by the authors. The authors alone are responsible for the content and writing of the paper, and report no conflicts of interest.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW255970. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA686202, SRR13275797, and SAMN17108662, respectively.
Additional information
Funding
References
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Dowton M, Austin AD. 1999. Evolutionary dynamics of a mitochondrial rearrangement “hot spot” in the Hymenoptera. Mol Biol Evol. 16(2):298–309.
- Dowton M, Castro LR, Campbell SL, Bargon SD, Austin AD. 2003. Frequent mitochondrial gene rearrangements at the hymenopteran nad3-nad5 junction. J Mol Evol. 56(5):517–526.
- Ghahari H, Abd-Radou S, Sakenin H, Hedqvist KJ, Ostovan H. 2010. A contribution to some chalcidoid wasps (Hymenoptera) from Iran. J Biol Control. 24(1):17–21.
- Heraty JM, Burks RA, Cruaud A, Gibson GAP, Liljeblad J, Munro J, Rasplus JY, Delvare G, Jansta P, Gumovsky A, et al. 2013. A phylogenetic analysis of the megadiverse Chalcidoidea (Hymenoptera). Cladistics. 29(5):466–542.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Meng GL, Li YY, Yang CT, Liu SL. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63.
- Oliveira DC, Raychoudhury R, Lavrov DV, Werren JH. 2008. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol Biol Evol. 25(10):2167–2180.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Sharkey MJ, Carpenter JM, Vilhelmsen L, Heraty J, Liljeblad J, Dowling AP, Schulmeister S, Murray D, Deans AR, Ronquist F, et al. 2012. Phylogenetic relationships among superfamilies of Hymenoptera. Cladistics. 28(1):80–112.
- Wang Y, Li CD, Zhang YZ. 2013. A taxonomic study of Chinese species of the alberti group of Metaphycus (Hymenoptera, Encyrtidae). ZooKeys. 285:53–88.
- Wolstenholme DR. 1992. Genetic novelties in mitochondrial genomes of multicellular animals. Curr Opin Genet Dev. 2(6):918–925.
- Xie R, Wu B, Dou H, Liu C, Knox GW, Qin H, Gu M. 2020. Feeding preference of crapemyrtle bark scale (Acanthococcus lagerstroemiae) on different species. Insects. 11(7):399.
- Xiong M, Zhou QS, Zhang YZ. 2019. The complete mitochondrial genome of Encyrtus infelix (Hymenoptera: Encyrtidae). Mitochondrial DNA Part B. 4(1):114–115.