Abstract
The complete mitochondrial genome of Fieberiella septentrionalis was annotated for the first time in the present study. The mitogenome was found to have circular shape, with 16,175 bp in size, containing 13 protein coding genes (PCGs), 22 transfer-RNA genes, 2 ribosomal-RNA genes, and 1 non-coding region. The nucleotide composition biases toward A and T is 77.9% of the entirety, which is a typical structure of Cicadellidae. All PCGs have ATN as the start codon, TAA and single T as the stop codon. The resulting phylogenetic tree confirms that the F. septentrionalis belongs to the subfamily of Deltocephalinae and Fieberiellini as sister to the remaining tribes of this subfamily.
Deltocephalinae is the largest and most economically important subfamily of leafhoppers, presenting distinct diagnostic characteristics and including over 6600 described extant species and 39 tribes (Zahniser and Dietrich Citation2013). Among the phylogenetic studies of the group, the taxon and character sampling in the morphological analysis of Deltocephalinae by Zahniser and Dietrich (Citation2008) is the most comprehensive to date. Fieberiella septentrionalis Wagner (Citation1963) belongs to Fieberiellini of the subfamily. It is distributed in Palearctic, Nearctic, and African, and often parasitizes dicotyledon trees and shrubs. It was first discovered by Wanger (1963) in Germany, but there is no information about the collection site and phylogenetic relationship in their article. The complete mitochondrial genome of F. septentrionalis reported in this article is for further study of its taxonomy and constructed phylogenetic tree to further validate taxonomic status on genome level.
In this study, we sequenced and annotated the complete mitochondrial DNA of the F. septentrionalis for the first time. The specimen of F. septentrionalis was collected in Shihezi city, Xinjiang Uygur Autonomous Region, China (N44.3122, E86.0569), in September 2019. Fresh specimens (voucher number IMNU20190908) were initially preserved in 100% ethanol, and then stored at −20 °C in the laboratory. After morphological identification, the entire body without abdomen was shipped to Tsingke (Beijing, China) for genomic extraction. The qualified genomic DNA is fragmented by mechanical interruption (ultrasonic), then the fragment is purified, end repaired, connected to the sequencing adapter, and selected by agarose gel electrophoresis. The library with insert size of 300 bp fragments was constructed using the PCR amplification and then sequenced was performed on the Illumina HiSeq 2000 instrument. The number and quality of raw paired-end reads were evaluated by using the FastQC (Andrews Citation2018). De novo assembly of clean reads was performed using SPAdes v3.11.0 (Bankevich et al. Citation2012). The mitogenome of Macrosteles quadrilineatus (GenBank accession number KY645960) was further used as a reference to assemble the sequenced sample. The mitochondrial genome was annotated with Geneious 9.1.4. All 13 protein-coding genes and 2 rRNA genes were determined by comparison with the homologous sequences of other leafhoppers from GenBank. The 22 tRNA genes were identified by MITOS WebServer (Bernt et al. Citation2013). The annotated sequence of F. septentrionalis mitogenome was deposited in GenBank with an accession number MW078430.
The mitochondrial genome of F. septentrionalis is 16,175 bp in length, with the A + T content of 77.9% (T 35.4%, C 12.6%, A 42.5%, and G 9.5%), which is a typical structure of Cicadellidae mitogenome (74–85%; Chen et al. Citation2020). Annotation of the mitogenome revealed 13 protein-coding genes (PCGs) (COX1-3, ND1-6, ND4L, ATP6, ATP8, and Cytb), 22 transfer-RNA (tRNA) genes, two ribosomal RNA unit genes (rRNAs) and one A-T rich region (Control region). Its gene arrangement and direction are in accordance with other Cicadellidae leafhopper (Boore Citation1999). Across the 13 PCGs in F. septentrionalis, only four genes (ND4, ND4L, ND5, and ND1) are coded on the minority strand (N-strand), whereas the others are coded on the majority strand (J-strand). Generally, PCGs are started with ATN codon and terminate with the stop codons TAA and TAG except for COX2 ended with single T. The 16S rRNA gene is 1224 bp in length and is located between tRNA-L1 and tRNA-V; the 12S rRNA gene is 755 bp in size and is located between tRNA-V and A-T-rich region. The A-T region is 1727 bp in size and located after the 12S rRNA.
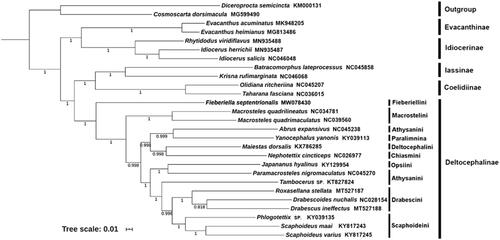
Here, we reconstructed a phylogeny of Cicadellidae using 13 PCGs from F. septentrionalis and other 24 representatives from 5 subfamilies of the family and 2 outgroup species from Cercopoidea and Cicadoidea (). Thirteen concatenated PCG sequences of mitogenomes were analyzed by the Bayesian inference (BI) method in MrBayes 3.2.6 (Ronquist et al. Citation2012). The optimal partitioning scheme and nucleotide substitution model for Bayesian inference (BI) phylogenetic analyses based on the nucleotide dataset of 13 PCGs were selected with PartitionFinder 2.1.1 (Lanfear et al. Citation2016) incorporated into PhyloSuite v1.2.1, using the branch lengths linked, Bayesian information criterion (BIC) model and the greedy search algorithm (Lanfear et al. Citation2012). The phylogenetic tree showed that Deltocephalinae was a monophyletic group in Cicadellidae and Fieberiellini was sister of the remaining tribes of this subfamily.
Acknowledgments
The authors thank Miss Xian Zhou (Northwest A&F University) for her assistance in analyzing the data.
Disclosure statement
No potential conflict of interest was reported by the authors. The authors alone are responsible for the content and writing of this article.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the Accession no. MW078430. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA705294, SRP13838339, and SAMN18080047, respectively.
Additional information
Funding
References
- Andrews S. 2018. Fast QC: a quality control tool for high throughput sequence data. [accessed 2020 March 8]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Boore JL. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27(8):1767–1780.
- Chen XX, Yuan ZW, Yuan XW, Song YH. 2020. Advances in mitochondrial genome complete sequence structure of leafhopper. Jiyinzuxue Yu Yingyong Shengwuxue (Genomics and Applied Biology). 39(06):125–137.
- Lanfear R, Calcot B, Ho SY, Guindon S. 2012. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 29(6):1695–1701.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2016. Partition Finder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34:772–773.
- Ronquist F, Teslenko M, Van P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Wagner W. 1963. Revision der europäischen Arten dreier Gattungen der Homoptera-Cicadina Dryodurgades Zakhvatkin, Fieberiella Signoret und Phlepsius Fieber. Entomologische Mitteilungen Aus Dem Zoologischen Staatsinstitut Und Zoologischen Museum Hamburg. 2(45):423–435.
- Zahniser JN, Dietrich CH. 2008. Phylogeny of the leafhopper subfamily Deltocephalinae (Insecta: Auchenorrhyncha: Cicadellidae) and related subfamilies based on morphology. Syst Biodivers. 6(1):1–24.
- Zahniser JN, Dietrich CH. 2013. A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur J Taxon. 45:1–121.