Abstract
The difficulties involved in the study of deep-sea species imply the existence of a wide gap in knowledge of this ecosystem. Here, we have obtained the mitogenome of the Oven's halosaur, Halosaurus ovenii, for the first time using low coverage Illumina Paired-end (PE) sequencing. This species belongs to order Notacanthiformes, a poorly studied group of deep-sea fishes. Moreover, given their evolutionary placement, they are critical to investigate the early diversification of the Teleostei. The assembled mitogenome displays the expected gene arrangement for vertebrate mtDNA. Phylogenetic analyses combining all the available mitogenomes of the Order Notacanthiformes were performed. The evolutionary relationships among H. ovenii and the rest of the Halosauridae family were confirmed. This mitogenome provides a valuable baseline for future research of H. ovenii.
The Oven's halosaur, Halosaurus ovenii Johnson, 1864, is a deep-sea marine fish species belonging to the family Halosauridae, a group of eel-shaped fish found worldwide but mainly distributed in the Atlantic Ocean (Bañón et al. Citation2016). Only three mitogenomes of halosaurid species have been published and none from the genus Halosaurus. In general, the economic costs related to deep-sea research limits the amount of knowledge related to these species. The mitogenome submitted in this manuscript is the first step toward a better understanding of the biology of this species.
The specimen was captured north of the Iberian Peninsula (42.2625 N; −9.4869 W) at 810.5 meters of depth during the DEMERSALES campaign 2019 and several tissue samples were stored in ethanol. Morphological identification was performed on board and later confirmed by means of COI mtDNA barcoding fragment. The specimen is stored at the Interdisciplinary Centre of Marine and Environmental Research (specimen code NOTAC0001).
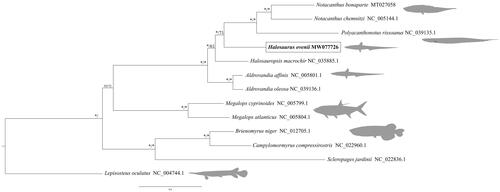
Whole genomic DNA was obtained from a small portion of the fin tissue using Qiagen MagAttract HMW DNA extraction kit. Library preparation (i.e. 350 bp insert) and sequencing (i.e. 150 bp Paired-end reads) were performed by Novogene Europe. Mitogenome assembly and annotation were obtained using SPAdes v3.12.0 (Bankevich et al. Citation2012) and MitoZ v.2.3 (Meng et al., Citation2019), respectively. All available mitogenomes of notacanthiform fish as well as six outgroup taxa were retrieved from GenBank (accessed in September 2020, ). Gene alignments of the 13 protein-coding genes (PCG) were produced using GUIDANCE2 (Sela et al. Citation2015) with MAFFT v 7.304 (Katoh and Standley Citation2013) and subsequently concatenated using FASconCAT-G (https://github.com/PatrickKueck/FASconCAT-G), resulting in a final alignment with 11 416 nucleotides. PartitionFinder2 (Lanfear et al. Citation2016) was used to estimate the best partition schemes and molecular evolutionary models for the phylogenetic Bayesian inference with MrBayes v3.2.6 (Ronquist et al. Citation2012), where two independent runs with four chains each were performed (107 generations, sampling one tree for every 1000 generations). Partition schemes and molecular evolutionary models for Maximum Likelihood phylogenetic inference were obtained using IQ-TREE v.1.6.12 (Nguyen et al. Citation2015; Kalyaanamoorthy et al. Citation2017). The H. ovenii complete mitogenome has been deposited in GenBank under the accession number MW077726. The total length of the assembled mitogenome is 16,647 bp, within the length observed on other species of the family, i.e., Halosauropsis macrochir 16,655 bp; Aldrovandia affinis 16,649 bp; and Aldrovandia oleosa 16,653 bp. Gene content and orientation are the same as the other Halosauridae mitogenomes available: 13 PCGs, 22 transfer RNA (trn), 2 ribosomal RNA (rrn) genes. NAD6 and 8 tRNAs are encoded on the light strand while the remaining genes are encoded on the heavy strand. The consensus tree obtained showed the relationships among the notacanthiform species (). The mitogenome of H. ovenii is closely related to other Halosauridae species and located before the common ancestor of the Notacanthide family. The values of pairwise p-distances among H. ovenii and the other Halosauridae vary from a minimum of 13.7% with H. macrochir and a maximum of 14.2% with A. oleosa. Overall, this information will be useful to a better understanding of the biology of deep-sea species.
Figure 1. Consensus tree of 13 mitogenomes, including seven notacanthiform species, obtained with BI and ML. Node values indicate posterior probabilities and bootstrap supports, respectively, with values above 95% represented with asterisks (*). The new mitogenome of Halosaurus ovenii is highlighted in bold.
Acknowledgments
We thank all the participants and crew of cruise Demersales19 performed on board the R/V Miguel Oliver.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
The data produced in this study are available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, reference number MW077726. Raw data can be made available from the corresponding author; Barros-García D.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Bañón R, Arronte JC, Armesto A, Barros-García D, de Carlos A. 2016. Halosaur fishes (Notacanthiformes: Halosauridae) from Atlantic Spanish waters according to integrative taxonomy. Zootaxa. 4184 (3):490.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2016. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34:msw260.
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63.
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Sela I, Ashkenazy H, Katoh K, Pupko T. 2015. GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 43(W1):W7–W14.
