Abstract
The graphic beauty butterfly Baeotus beotus (Doubleday 1849) (Nymphalidae: Coeini) is a rare fruit feeding species that inhabits humid tropical forests in Central and South America. Genome skimming by Illumina sequencing allowed assembly of a complete circular mitogenome of 15,131 bp from B. beotus consisting of 80.4% AT nucleotides, 22 tRNAs, 13 protein-coding genes, two rRNAs and a control region in the typical butterfly gene order. Baeotus beotus COX1 features an atypical CGA start codon and COX1, COX2, ND1, and ND4 exhibit incomplete stop codons completed by the addition of 3’ A residues to the mRNA. Bayesian phylogenetic reconstruction places B. beotus as sister to Cyrestis thyodamas (Cyrestini), which is consistent with previous phylogenetic hypotheses.
The graphic beauty, Baeotus beotus (Doubleday, 1849) is a rare fruit feeding butterfly which inhabits humid tropic forests in Central and South America (Warren et al. Citation2012; van den Berghe et al. Citation2016). This South American species is sexually dimorphic and its larval hosts plants are still unknown (van den Berghe et al. Citation2016). It was originally placed in Megistansis (Charaxinae), but this genus was later discovered to be invalid as it was already in use for the type specimen of Papilio cadmus (Crammer, 1775) so a new genus was created for this species and its congeners (Hemming Citation2009). The genus Baeotus includes four described species (B. aeilus, B. baeotus, B. deucalion, B. japetus) with maximum biodiversity in the upper Amazon Basin, but the ranges of some Baeotus species extend as far north as southeastern Mexico (Warren et al. Citation2012). Here, I report the complete mitochondrial genome sequence of B. beotus from specimen BB2019.1, collected in Tingo Maria, Peru (GPS 9.29616S, 75.99831 W) in January 2019 that has been pinned, spread, and deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (http://www.wallisroughley.ca/, Dr. Jason Gibbs, [email protected]) voucher WRME0507736.
DNA was prepared (McCullagh and Marcus Citation2015), then later sequenced by Illumina NovaSeq6000 (San Diego, CA) (Marcus Citation2018). The mitogenome of B. beotus (Genbank MW566598) was assembled and annotated by Geneious 10.2.6 from an SRA library of 25,452,563 paired 150 bp reads (Genbank SRA PRJNA699171) using a Protogoniomorpha ancardii duprei reference mitogenome (Lepidoptera: Nymphalidae, MT702382) (Lalonde and Marcus Citation2020). The B. beotus nuclear rRNA repeat (Genbank MW571038) was also assembled and annotated using a P. ancardii duprei (MT702383) reference sequence.
The B. beotus circular 15,131 bp mitogenome assembly was composed of 11,656 paired reads with nucleotide composition: 39.8% A, 11.9% C, 7.7% G, and 40.6% T. The gene composition and order in B. beotus matches the typical arrangement found in most butterfly mitogenomes (Park et al. Citation2016). The B. beotus protein coding gene start codons include: ATG (ATP6, COX2, COX3, CYTB, ND1, ND4, ND4L), ATT (ATP8, ND3, ND5, ND6), ATC, (ND2), and CGA, an atypical COX1 start codon that is also found in the COX1 gene of many other insects (Liao et al. Citation2010). The mitogenome contains three protein-coding genes (COX1, COX2, ND4) with single-nucleotide (T) stop codons, and one protein-coding gene (ND1) with two-nucleotide (TA) stop codons completed by post-transcriptional addition of 3′ A residues. The structures of tRNAs were determined using ARWEN v.1.2 (Laslett and Canback Citation2008) and have typical cloverleaf secondary structures except for trnS (AGN) where the dihydrouridine arm is replaced by a loop, while the mitochondrial rRNAs and control region are typical for Lepidoptera (McCullagh and Marcus Citation2015).
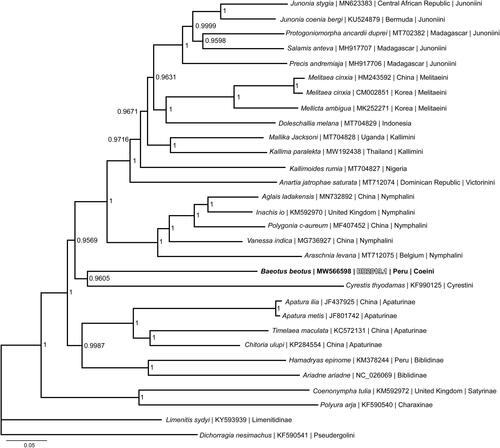
Phylogenetic reconstruction was done using the complete mitogenome of B. beotus, 26 mitogenomes from the subfamily Nymphalinae, and three outgroup species (Satyrinae (Coenonympha tulia), Charaxinae (Polyura arja), and Limenitidinae (Limenitid sydyi)) from other subfamilies within the Nymphalidae (Wahlberg et al. Citation2005; Alexiuk et al. Citation2020; Hamilton et al. Citation2020; Payment et al. Citation2020) (). Mitogenome sequences were aligned in CLUSTALX 2.1 (Thompson et al. Citation1997; Larkin et al. Citation2007) and analyzed using Bayesian Inference with the GTR + I+G model (model selected by jModeltest 2.1.1 (Darriba et al. Citation2012)) in Mr. Bayes version 3.2.7 (Ronquist and Huelsenbeck Citation2003; Ronquist et al. Citation2012). Phylogenetic analysis places B. beotus from the tribe Coeini as the sister taxon to Cyrestis thyodamas (Cyresini), which is consistent with recent phylogenetic analyses (Wahlberg et al. Citation2005; Kodandaramaiah and Wahlberg Citation2007). One unexpected outcome of the phylogenetic reconstruction was the mitogenome from the representative of Nymphalinae tribe Pseudergolini (Dichorragia nesimachus) was found to the group outside of subfamily Nymphalinae, which is inconsistent with other recent molecular phylogenetic reconstructions (Wahlberg et al. Citation2005, Citation2009). Further phylogenetic investigation of tribe Pseudergolini may be required to determine its taxonomic relationship to other members of subtribe Nymphalinae.
Figure 1. Bayesian Inference phylogeny (GTR + I+G model, marginal likelihood score 135,267.87) of the Baeotus beotus mitogenome, 26 additional mitogenomes from tribes within subfamily Nymphalinae, and three outgroup species (Satyrinae (Coenonympha tulia), Charaxinae (Polyura arja), and Limenitidinae (Limenitid sydyi)) from other subfamilies within family Nymphalidae produced by 1.5 million iterations in MrBayes with sampling every 100 generations. The Bayesian posterior probability values determined by MrBayes are given at each node.
Acknowledgments
I would like to thank Jeffrey Marcus for his constructive criticism of this manuscript and Genome Quebec for assistance with library preparation and sequencing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, reference numbers PRJNA699171, MW566598, and MW571038.
Additional information
Funding
References
- Alexiuk MR, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the Jackson’s leaf butterfly Mallika jacksoni (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 5:3316–3318.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772.
- Hamilton RV, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the black dead leaf butterfly Doleschallia melana (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 5:3306–3308.
- Hemming F. 2009. Notes on the generic nomenclature of the lepidoptera Rohpalocer, I. Syst Ent. 8(7):133–138.
- Kodandaramaiah U, Wahlberg N. 2007. Out-of-Africa origin and dispersal-mediated diversification of the butterfly genus Junonia (Nymphalidae: Nymphalinae). J Evol Biol. 20(6):2181–2191.
- Lalonde MML, Marcus JM. 2020. The complete mitochondrial genome of the Malagasy clouded mother-of-pearl butterfly Protogoniomorpha ancardii duprei (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 5:3261–3263.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23(21):2947–2948.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6(2):172–186.
- Marcus JM. 2018. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 5(1):1–23.
- McCullagh BS, Marcus JM. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae. J Asia-Pacific Ent. 18(4):749–755.
- Park JS, Kim MJ, Jeong SY, Kim SS, Kim I. 2016. Complete mitochondrial genomes of two gelechioids, Mesophleps albilinella and Dichomeris ustalella (Lepidoptera: Gelechiidae), with a description of gene rearrangement in Lepidoptera. Curr Genet. 62(4):809–826.
- Payment JE, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the African leaf butterfly Kallimoides rumia (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 5:3415–3417.
- Ronquist F, Huelsenbeck JP. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19(12):1572–1574.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25(24):4876–4882.
- van den Berghe E, Hernandez B, Perez Vasques ME, Orozco A. 2016. Baeotus beotus (Doubleday, 1849) (Lepidoptera: Charaxinae) nuevo para la Fauna de Nicaragua. Revista Nicaraguense de Entomologia. 102:3–9.
- Wahlberg N, Brower AVZ, Nylin S. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol J Linn Soc Lond. 86(2):227–251.
- Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc Biol Sci. 276(1677):4295–4302.
- Warren AD, Davis KJ, Grishin NV, Pelham JP, Stangeland EM. 2012. Interactive listing of American butterflies. [30-XII-12] http://www.butterfliesofamerica.com/.
