Abstract
Omosita colon (Linnaeus, 1758) (Coleoptera: Nitidulidae) is an economically important storage pest worldwide and a forensically important beetle. The first complete mitochondrial genome (mitogenome) of O. colon was sequenced in this study using the next-generation sequencing. The mitogenome of O. colon is circular with a total length of 16,544 bp, which consists of 13 protein-coding genes, 22 transfer RNA genes, two ribosomal RNA genes, and a non-coding control region. The order and orientation of genes were identical with that of the ancestral insects. This study provides genomic data for mitogenome library of the genus Omosita to investigate evolutionary and systematic studies. It also provides a molecular basis to infer the postmortem interval (PMImin) with O. colon.
1. Introduction
The genus Omosita (Coleoptera: Nitidulidae: Nitidulinae) is a group of Nitidulidae, which is widely distributed in the Holarctic regions (Lee et al. Citation2015). Some species are considered as important stored-product pests. In this family, Omosita colon (Linnaeus, 1758) (Coleoptera: Nitidulidae) is not only a pest of stored Chinese herbal medicines and stored grain products but also a forensically important necrophagous beetle. O. colon is closely associated with corpses () and has been reported in many insect fauna succession studies (De Jong and Hoback Citation2006; Anton et al. Citation2011; Matuszewski et al. Citation2013; Lyu et al. Citation2016). In China, it is widely distributed in most of the provinces and districts (Zhang et al. Citation2008).
Being an important insect, there have been very few studies on O. colon. Most of them focused only on the taxonomy of O. colon (Cao and Huang Citation2016; Jelinek and Audisio Citation2009). Beyond that, few other aspects of O. colon have also been reported. For example, Wang et al. (Citation2020) studied the development of O. colon at seven constant temperatures between 16 and 34 °C, which provided fundamental development data that supported use of O. colon in minimum postmortem interval (PMImin) estimations. Molecular information on O. colon is inadequate, and only a few reports mentioned about the taxonomic study of beetle species in certain areas (Pentinsaari et al. Citation2014; Hendrich et al. Citation2015). On commencement of the study, there were only 13 partial gene sequences of O. colon in GenBank, and the characterization of the mitochondrial genome (mitogenome) of O. colon has not been conducted till date. However, the molecular data of O. colon is considered significant as it provides the basis for the taxonomic study of beetles. On the other hand, it establishes the molecular basis for PMImin estimation with O. colon in the field of forensic medicine. Therefore, it is not only of scientific significance but also of practical application value to study the mitogenome of O. colon.
Mitogenome is a common molecular marker for evolutionary, phylogenetic, and population genetic studies, which provides information for analyses of several taxonomic levels (Drosopoulou et al. Citation2019). Besides, when molecular identification of a species is required, it is helpful to select the most suitable mitochondrial marker by analyzing the complete mitogenome. The structure of insect mitogenome is relatively conservative consisting of 37 genes: 13 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, and two ribosomal RNA (rRNA) genes (Song et al. Citation2016; Feng et al. Citation2018). Recently, the mitogenome has been widely used in the phylogenetic studies (Burger et al. Citation2003; Charles Citation2005). The genome organization, gene arrangement, preference of the codons, and tRNA structure in the mitogenome can be used in the phylogenetic tree construction (Qiang et al. Citation2018; Wang et al. Citation2018). So far, no studies have been conducted on mitogenome of the genus Omosita. In this study, the complete mitogenome of O. colon was sequenced and assembled by next-generation sequencing. In conclusion, we report the first complete mitogenome of O. colon, along with analysis of its gene arrangement, and phylogenetic analysis of nitidulid beetles. This study provides a complete genomic data for mitogenome library of the genus Omosita to investigate both evolutionary and systematic studies, and to provide a molecular basis to infer the PMImin with O. colon.
2. Materials and methods
2.1. Insects
The O. colon specimens used in this study were collected from a pig carcass placed in a field environment in Suzhou, China (31°21′N, 120°53′E) in April 2018. Species identification was conducted under a Zeiss 2000-C stereomicroscope (Jena, Germany) according to the identification keys provided by Zhang et al. (Citation2008). The captured adults were reared under a constant temperature incubator for further study.
2.2. DNA extraction, sequencing, and assembly
Total genomic DNA was extracted from single O. colon using Rapid Animal Genomic DNA Isolation Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s instructions for total DNA purification from animal tissues. 1% agarose gel electrophoresis (voltage: 200 V, time: 30 min) was used to detect DNA integrity, and Qubit was used to detect the concentration of DNA samples.
On obtaining the total DNA of O. colon, we used DNA Library Prep Kit from Illumina (NEB, Ipswich, MA) for library preparation. Sequencing was constructed on Illumina Hiseq2500 Platform with HiSeq PE150 mode (Paired-end) (Sangon Biotech, Shanghai, China).
Prior to quality control, the statistical information of BBtools was used to evaluate the quality of the original data, and some basic information was counted and visualized to determine the data quality. The raw data was then quality-controlled using BBduk and BLAST+. After that, we used the NOVOPlasty software for de novo assembly of the mitogenome of O. colon with the obtained clean reads. If the result was not desirable, we extracted the mapped reads, used Spades version 3.13.0 (St Petersburg, Russia) for splicing, and tried to choose the appropriate contig connection mode through Blast+.
2.3. Annotation and analysis
The genes of the mitogenome were predicted using MITOS2 Server (http://mitos.bioinf.uni-leipzig.de/index.py) (Bernt et al. Citation2013). Then, tRNA genes were predicted using tRNAscan-SE version 1.3.1 (California, USA) (Lowe and Chan Citation2016) and ARWEN (Laslett and Canb Citation2008). Meanwhile, MEGA version 7.0 (Auckland, New Zealand) (Kumar et al. Citation2016) was used to analyze the nucleotide composition, and relative synonymous codon usage (RSCU) was analyzed by Biopython. Strand asymmetry of the mitogenome was assessed using the following formulas: AT skew = [A − T]/[A + T], and GC skew = [G − C]/[G + C] (Junqueira et al. Citation2004). Finally, the circular map of the complete mitogenome was drawn with OGDRAW (Lohse et al. Citation2013).
We used data from the newly sequenced mitogenome of O. colon and those of 14 other taxa for phylogenetic analysis of the infraorder Cucujiformia (Coleoptera and Polyphaga). As outgroups, we used one species from the infraorder Scirtiformia (). The bootstrap consensus tree was inferred by the maximum-likelihood (ML) method based on the Kimura 2-parameter model with 1000 bootstrap replicates. All the above alignments, analyses, model selection, and phylogeny reconstruction were performed in MEGA version 10.0 (Auckland, New Zealand).
Table 1. GenBank accession numbers of published infraorder Cucujiformia members and outgroup.
3. Results
3.1. Mitogenome organization and nucleotide composition
After assembly, the complete mitogenome of O. colon has been found to be 16,544 bp in length, which can be assembled into one circular contig (). The mitogenome of O. colon is similar to other nitidulid beetles in terms of gene composition (Wu et al. Citation2019), containing 22 tRNA genes, 13 PCGs, two rRNA genes, and a non-coding region (). In addition, they also shared parallel gene arrangement: eight tRNA genes (trnC, F, H, L2, P, Q, V, and Y), four PCGs (ND1, ND4, ND4L, and ND5), and two rRNA genes (l-rRNA and s-rRNA) were located in the light strand, while the other genes were located in the heavy strand.
Figure 2. The mitogenome arrangement of O. colon. The red color is transfer RNA (tRNA) genes, the green is protein-coding genes (PCGs), and the orange is ribosomal RNA (rRNA) genes.
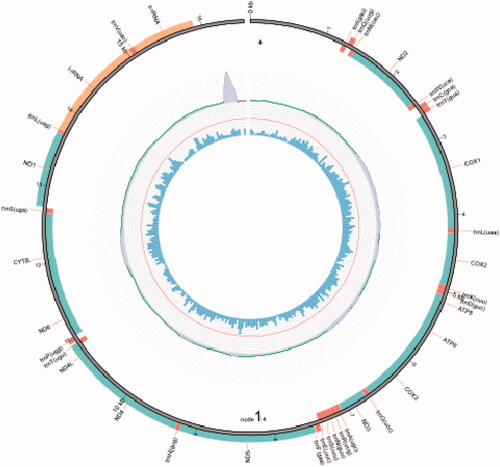
Table 2. Organization of the O. colon mitogenome.
The total length of the intergenic sequences in O. colon is 43 bp, with the longest intergenic sequence located between trnS2 (uga) gene and ND1 gene with 17 bp length. Furthermore, the total length of the overlapping regions in O. colon is 97 bp. All the overlapping sequences of the mitogenome of O. colon range from 1 to 38 bp, and the longest overlapping sequence is located between trnL2 (uag) gene and l-rRNA gene with 38 bp. Compared to the mitogenomes of other nitidulid beetles, the total length of the intergenic sequences in O. colon seemed to be much shorter, and the total length of the overlapping sequences was similar (Wu et al. Citation2019).
The nucleotide composition of O. colon is A: 41.1%, T: 38.7%, G: 8.3%, and C: 11.9% (). Compared to the mitogenomes of other insect species, the nucleotide composition of O. colon is also biased on A and T composition (A + T: 79.8%). Furthermore, AT-skew of the mitogenome is slightly positive with a value of 0.029 while GC-skew is negative −0.178 for O. colon. In most of the metazoan mitogenomes, the strand skew biases are found to be weakly positive AT-skew and strongly negative GC-skew (Li et al. Citation2017), which is found in this study as well in case of O. colon.
Table 3. Nucleotide composition of O. colon mitogenome.
3.2. Protein coding genes and codon usage
The size of 13 PCGs is 11,193 bp in the O. colon mitogenome, with 33 bp for stop codons. The majority of PCGs are encoded by the H strand and only ND1, ND4, ND4L, and ND5 are encoded by the L strand as shown in . Three types of start codons – ATT, ATC, and ATG – are used, of which ATT is the most common used start codon, and only ND2 gene starts with ATC. Additionally, there are four types of stop codon: TAA, TAG, TA, and T, of which TAA is the most commonly used, while CO II, CO III, ND5, and ND4 genes stop with incomplete stop codon TA or T. Incomplete stop codons are common in animal mitochondrial DNA and are likely to be completed by post-transcriptional polyadenylation (Ojala et al. Citation1981).
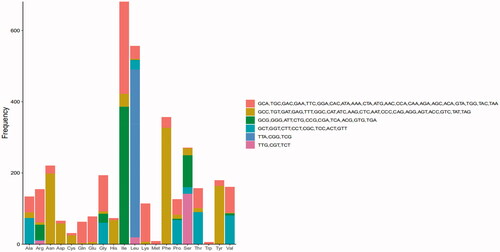
shows the RSCU of the O. colon mitogenome. Codon usage analysis indicated that the most frequently used codons in the O. colon mitogenome were TAA, ATT, TTT, ATA, and AAT. The frequent use of A and T in codon contributed to the high AT content in the O. colon mitogenome.
3.3. tRNA and rRNA genes
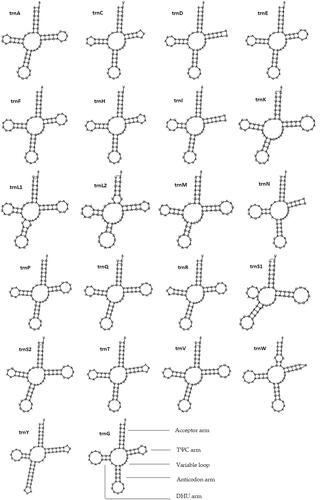
There are 22 tRNA genes in the mitogenome of O. colon, which are dispersed among the PCGs and rRNA genes. Among them, eight tRNAs lie on L strand, and 14 tRNAs lie on H strand. The positions and sizes (62–72 nucleotides) of tRNAs in the mitogenome of O. colon follows the typical organization for insect mtDNA.
Most tRNAs could be folded into the clover-leaf secondary structures including the aminoacyl (or acceptor) arm, dihydrouridine (DHU) arm, anticodon arm, and pseudouridine (TΨC) arm, except for trnD, trnI, trnN, and trnW, which lacked the TΨC-loop; meanwhile, trnS1 lacked the DHU arm ().
The l-rRNA gene of O. colon mitogenome consists of 1334 nucleotides (position: 13714 − 15047), and the s-rRNA gene consists of 787 nucleotides (position: 15113–15899). In accordance with other insect mitogenomes, rRNA genes in the O. colon mitogenome are located in the L strand between the trnL2 (uag) gene and the control region, separated by the trnV gene.
3.4. Phylogenetic analysis
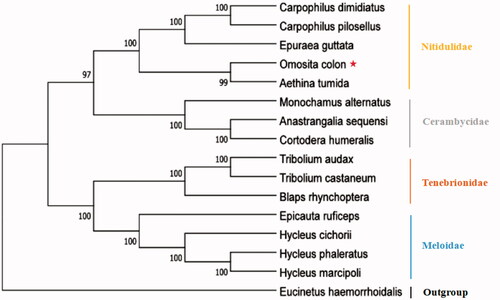
The ML phylogenetic analysis with 15 complete mitogenomes (one generated in this study and 14 obtained from the GenBank) was conducted with MEGA version 10.0 (). As shown in the figure, the clustering results of each branch were consistent with those of the taxonomy. Phylogenetic analysis results strongly supported that O. colon was closely related to Aethina tumida. The results indicate that O. colon and four other species of Nitidulidae form a clade, and that the monophyly of the family is well recovered.
4. Discussion
The complete mitogenome data is considered useful for genetic identification and phylogenetic studies. Furthermore, O. colon is an economically important storage pest worldwide and a forensically important beetle as well. In this study, we reported the first complete mitogenome sequencing of O. colon, and the result provided a molecular basis for species identification and inferring the PMImin with O. colon.
We compared the mitogenomes of O. colon and other nitidulid beetles with respect to AT/GC contents, mitogenome organization, and codon usage patterns. We found that most of these features were similar, which meant that the gene order and other structural features of nitidulid beetles were largely conserved. In addition, we observed that the mitogenome of O. colon was very similar to other Coleoptera species in terms of gene organization, order, and size. For example, the length of Dermestes tessellatocollis (Coleoptera: Dermestidae) mitogenome is 16,218 bp, containing 13 PCGs, two rRNAs, and 22 tRNAs as well (Karagozlu et al. Citation2019). The similarities between these mitogenomes might be considered as common characteristics in case of Coleoptera species.
The arrangement of mitochondrial genes is an important reference for revealing the phylogenetic relationships among the species (Bo-Ying et al. Citation2018). The order of mitochondrial genes in animals has been extensively studied, and some models have been proposed to explain this rearrangement (Marleen et al. Citation2008). In this study, the first mitogenome sequence for species within the genus Omosita was reported, and more data from other species in this genus will be needed for carrying out further research. Mitogenome sequences will enable the resolution of species identification, phylogenetics studies, and molecular evolution of family Nitidulidae (Wu et al. Citation2019), which would supplement further researches in similar field.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Mitogenome data supporting this study are openly available in GenBank at: https://www.ncbi.nlm.nih.gov/nuccore/MT749275. Associated BioProject, SRA, and BioSample accession numbers are https://www.ncbi.nlm.nih.gov/bioproject/PRJNA670574 https://www.ncbi.nlm.nih.gov/sra/SRR12879478 and SAMN16513378, respectively.
Additional information
Funding
References
- Anton E, Niederegger S, Beutel RG. 2011. Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med Vet Entomol. 25(4):353–364.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Bo-Ying Z, Li-Jun C, Pu T, Kees VA, Ary AH, Hua-Yan C, Xue-Xin C, Shu-Jun W. 2018. Gene arrangement and sequence of mitochondrial genomes yield insights into the phylogeny and evolution of bees and sphecid wasps (Hymenoptera: Apoidea). Mol Phylogenet Evol. 124:1–9.
- Burger G, Gray MW, Lang BF. 2003. Mitochondrial genomes: anything goes. Trends Genet. 19(12):709–716.
- Cao Y, Huang M. 2016. A SEM study of the antenna and mouthparts of Omosita colon (Linnaeus) (Coleoptera: Nitidulidae). Microsc Res Tech. 79(12):1152–1164.
- Charles EB, Franz Lang B. 2005. Fungal evolution: the case of the vanishing mitochondrion. Curr Opin Microbiol. 8:362–369.
- De Jong GD, Hoback WW. 2006. Effect of investigator disturbance in experimental forensic entomology: succession and community composition. Med Vet Entomol. 20(2):248–258.
- Drosopoulou E, Syllas A, Goutakoli P, Zisiadis G, Konstantinou T, Pangea D, Sentis G, van Sauers-Muller A, Wee S, Augustinos AA, et al. 2019. The complete mitochondrial genome of Bactrocera carambolae (Diptera: Tephritidae): genome description and phylogenetic implications. Insects. 10(12):429.
- Feng S, Yang Q, Li H, Song F, Stejskal V, Opit GP, Cai W, Li Z, Shao R. 2018. The highly divergent mitochondrial genomes indicate that the booklouse, Liposcelis bostrychophila (Psocoptera: Liposcelididae) is a cryptic species. Genome Biol Evol. 8:1039–1047.
- Hendrich L, Morinière J, Haszprunar G, Hebert PDN, Hausmann A, Köhler F, Balke M. 2015. A comprehensive DNA barcode database for Central European beetles with a focus on Germany: adding more than 3500 identified species to BOLD. Mol Ecol Resour. 15(4):795–818.
- Jelinek J ,Audisio P. 2009. The Kateretidae, Nitidulidae and Monotomidae (Coleoptera: Cucujoidea) described by Gistel (1856,1857): new synonymies and type designations. Acta Ent Mus Nat Pra. 49:225–238.
- Junqueira ACM, Lessinger AC, Torres TT, Da SFR, Vettore AL, Arruda P, Azeredo EAML. 2004. The mitochondrial genome of the blowfly Chrysomya chloropyga (Diptera: Calliphoridae). Gene. 339:7–15.
- Karagozlu MZ, An H, Park SH, Shin SE, Kim C. 2019. Comparative analyses of the three complete mitochondrial genomes from forensic important beetle genus Dermestes with phylogenetic relationships. Gene. 706:146–153.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
- Laslett D, Canb CBR. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Lee S, Kirejtshuk A, Lee S. 2015. Review of the genus Omosita Erichson (Coleoptera: Nitidulidae: Nitidulinae) in Korean fauna, with key to the Palaearctic species. J Asia-Pac Entomol. 18(4):837–843.
- Li X, Li W, Ding S, Cameron SL, Mao M, Shi L, Yang D. 2017. Mitochondrial genomes provide insights into the phylogeny of Lauxanioidea (Diptera: Cyclorrhapha). Int J Mol Sci. 18(4):773.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. Organellar GenomeDRAW-a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:W575–W581.
- Lowe TM, Chan PP. 2016. tRNAscan-SE on-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Lyu Z, Wan L, Yang Y, Tang R, Xu L. 2016. A checklist of beetles (Insecta, Coleoptera) on pig carcasses in the suburban area of southwestern China: a preliminary study and its forensic relevance. J Forensic Leg Med. 41:42–48.
- Marleen P, Guido F, Kai R, Matthias B, Daniel M, Martin M, Detlef B, Peter FS, Martin S. 2008. Evolution of mitochondrial gene orders in echinoderms. Mol Phylogenet Evol. 47:855–864.
- Matuszewski S, Szafa OM, Jarmusz M. 2013. Insects colonising carcasses in open and forest habitats of Central Europe: search for indicators of corpse relocation. Forensic Sci Int. 231(1–3):234–239.
- Ojala D, Montoya J, Attardi G. 1981. tRNA punctuation model of RNA processing in human mitochondria. Nature. 290(5806):470–474.
- Pentinsaari M, Hebert PD, Mutanen M. 2014. Barcoding beetles: a regional survey of 1872 species reveals high identification success and unusually deep interspecific divergences. PLoS One. 9(9):e108651.
- Qiang L, Cheng C, Chuan X, Xin J, Zuqin C, Wenli H. 2018. Comparative mitogenomics reveals large-scale gene rearrangements in the mitochondrial genome of two Pleurotus species. Appl Microbiol Biot. 102:6143–6153.
- Song F, Li H, Jiang P, Zhou X, Liu J, Sun C, Vogler AP, Cai W. 2016. Capturing the phylogeny of holometabola with mitochondrial genome data and bayesian site-heterogeneous mixture models. Genome Biol Evol. 8(5):1411–1426.
- Wang Y, Wang M, Hu G, Xu W, Wang Y, Wang J. 2020. Temperature-dependent development of Omosita colon at constant temperature and its implication for PMImin estimation. J Forensic Leg Med. 72:101946.
- Wang Z, Wang Z, Shi X, Wu Q, Tao Y, Guo H, Ji C, Bai Y. 2018. Complete mitochondrial genome of Parasesarma affine (Brachyura: Sesarmidae): gene rearrangements in Sesarmidae and phylogenetic analysis of the Brachyura. Int J Biol Macromol. 118:31–40.
- Wu Y, Lan Y, Xia L, Cui M, Sun W, Dong Z, Cao Y. 2019. The first complete mitochondrial genomes of two sibling species from Nitidulid beetles pests. Insects. 11(1):24.
- Zhang SF, Shi SF, Shi ZW, Xue GH. 2008. Atlas of beetles associated with stored products. 1st ed. Beijing, China: China Agriculture Press; p. 133.