Abstract
The genus Syringa (Oleaceae) comprises some of the most important cultivated horticultural trees worldwide. Syringa komarowii, is one of these important horticultural plants. We sequenced the complete chloroplast (cp) genomes of S. komarowii (previously identified as var. reflexa) using Illumina Hiseq X Ten platform. The cp genome exhibits a typical quadripartite structure with 158,020 bp in length, which consists of two copies of inverted repeat (IR) regions (25,676 bp) separated by a large single copy (LSC) region (87,628 bp) and a small single copy (SSC) region (19,040 bp). The cp genome of S. komarowii encodes a total of 132 genes, including 88 protein-coding genes, 36 tRNA genes and 8 rRNA genes. A maximum likelihood (ML) phylogenetic analysis resolved that S. komarowii in a clade with S. wolfii and S. yunnanensis. The ML tree also showed Syringa appeared more closely related to Ligustrum than to the other genera in the Oleaceae.
Syringa is distributed in China, North Korea, Japan and Southeast Europe. The species of Syringa have high ornamental, medicinal, economic and environmental value, and are an indispensable landscape ornamental plant. China has most of the wild resources of Syringa and is recognized as the natural distribution center for Syringa (Zang and Cui Citation2000). Syringa. komarowii, arguably has the best ornamental values in the genus Syringa, because of its contrasting pink outer corolla and white inner corolla. In this study, the complete chloroplast (cp) genome of S. komarowii, previously accepted with varietal status as S. komarowii var. reflexa, is reported to provide genomic data for future research.
Syringa komarowii var. reflexa was collected from Huoditang (located in the center of Qinling Mountains, is the teaching and experimental forest farm of Northwest A&F University) (36.32°N, 108.35°E). The samples were dried with silica gel and stored in the herbarium of Northwest A&F University (email: [email protected], Chaoyang Zhang) under the voucher number SY201913. DNA was extracted from leaf tissue using the Plant Genomic DNA kit (Tiangen Biotech, Beijing, China) following the modified Cetyl Trimethyl Ammonium Bromide method (Doyle Citation1987). The complete genome sequencing was performed using the Illumina HiSeq X Ten Platform (Illumina, San Diego, CA) with 350 bp pair-end reads on at Biomarker Technologies Inc. (Beijing, China). The high-quality paired-end reads were trimmed and assembled with MIRA version 4.0.2 (Chevreux et al. Citation2004) and MITObim-master version1.7 (Hahn et al. Citation2013) using the S. yunnanensis (Olofsson et al. Citation2019) chloroplast genome sequence as a reference (GenBank number NC_042468). Annotations were performed using Geneious R version 10.2.2 (Biomatters, Auckland, New Zealand). The chloroplast genome sequence was deposited in GenBank, accession number MT648823.
The cp genome of S. komarowii is 158,020 bp in length and contains one LSC of 87,628 bp and one SSC of 19,040 bp, which were separated by a pair of IRs of 25,676 bp. The guanine and cytosine content of the cp genome, LSC, SSC and IR regions were 37.9%, 36.2%, 32.6%, and 43.0%, respectively. In total, 132 genes were predicted in the plastid genomes of S. komarowii, including 8 rRNA, 36 tRNA, and 88 protein-coding genes. Among these genes, 14 genes (atpF, ndhA, ndhB, petB, petD, rpl2, rps12, rps16, rpoC1, trnI-GAU, trnA-UGC, trnK-UUU, trnG-GCC, and trnL-UAA,) have one intron, and two genes (ycf3 and clpP) contain two introns. The longest intron was trnK-UUU at 2,494 bp.
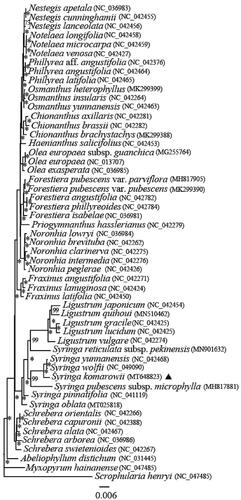
Phylogenetic trees were reconstructed using Maximum likelihood (ML) methods with cp genome sequences of 53 species. Scrophularia henryi was set as an outgroup to infer the phylogenetic positions of S. komarowii within Syringa and relationships of Oleaceae. All of the 53 cp genome sequences were aligned using MAFFT (Katoh and Standley Citation2013) with default parameter. To reconstruct the phylogenetic relations of these species, we used IQ-TREE 1.6.12 (Nguyen et al. Citation2015) with ML analysis, using the general time reversible (GTR) model by the program jModeltest version 2.1.7 (Darriba et al. Citation2012). The node stability was assessed using a rapid bootstrapping analysis with 1000 replicates. The phylogenetic tree indicated that S. komarowii was close to S. wolfii and S. yunnanensis (MLBS = 100%), Syringa closely related to Ligustrum (), which is consistent with previous molecular results (Li et al. Citation2002). The cp genome of S. komarowii provides a valuable resource for evolutionary research in the Oleaceae and also demonstrates the effectiveness of chloroplast phylogenomics to further resolve the phylogenetic relationships in the family.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MT648823. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA705853, SRR13823815, and SAMN18104659 respectively.
Additional information
Funding
References
- Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Müller WE, Wetter T, Suhai S. 2004. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 14(6):1147–1159.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772–772.
- Doyle JJ. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads - a baiting and iterative mapping approach. Nucleic Acids Res. 41(13):e129–e129.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Li JH, Alexander JH, Zhang DL. 2002. Paraphyletic Syringa (Oleaceae): Evidence from sequences of nuclear ribosomal DNA ITS and ETS regions. Syst Bot. 27:592–597.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating Maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Olofsson JK, Cantera I, Van de Paer C, Hong‐Wa C, Zedane L, Dunning LT, Alberti A, Christin P‐A, Besnard G. 2019. Phylogenomics using low-depth whole genome sequencing: a case study with the olive tribe. Mol Ecol Resour. 19(4):877–892.
- Zang SY, Cui HX. 2000. Syringa. Shanghai: Shanghai Scientific and Technical Publishers; p. 1–5.