Abstract
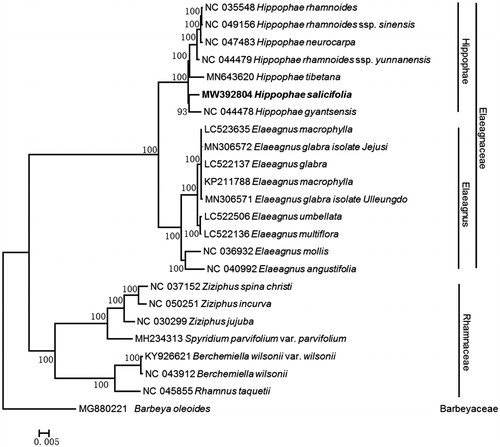
Seabuckthorn (Hippophae L.) is a pioneer species widely distributed in Eurasia. We assembled and annotated the chloroplast genome of Hippophae salicifolia from Illumina pair-end data, which was 155,420 bp in length with 36.74% GC content; this plastome featured a quadripartite structure with two copies of a large inverted repeat (IR) of 26,528 bp separated by large single copy (LSC) region of 83,504 bp and small single copy region (SSC) of 18,860 bp. In total, 131 complete genes were annotated, including 38 tRNA, eight rRNA, and 85 protein-coding genes. Phylogenetic analysis recovered H. salicifolia and H. gyantsensis as monophyletic and sister to all other Hippophae species for which complete plastome sequences have been published.
Genus Hippophae (Elaeagnaceae) consists of dioecious shrub or small tree species, distributed widely across Eurasia with a center of diversity in the Qinghai-Tibet Plateau (QTP) (Qin and Gilbert Citation2007). In northern China, seabuckthorn is widely used in sand fixation and preventing desertification. However, the phylogenetic relationships within Hippophae are controversial. Reconstructions based on five nuclear DNA regions supported a clade containing H. neurocarpa and H. salicifolia; based on five chloroplast genes; however, this clade has low support. Combined nuclear and plastid data resolved a clade composed of H. tibetana and H. rhamnoides with moderate to strong support (Jia and Bartish Citation2018). Nevertheless, the phylogenetic relationships among H. neurocarpa, H. gyantsensis, and H. tibetana based on 32 complete chloroplast genomes revealed H. rhamnoides was more closely related to H. neurocarpa than H. tibetana (Wang et al. Citation2018; Zhou et al. Citation2019; Zhou et al. Citation2020). Here, we sequenced and assembled the complete chloroplast genome of H. salicifolia, and compared with other Hippophae species.
The specimen of H. salicifolia was collected on the QTP in China (N 27.868339, E 91.800321). The voucher specimen was deposited in the herbarium of School of Life Sciences Fudan University (herbarium code: FUS, voucher number: HT0334, contact person: Ruoqiu Wang, email: [email protected]). Fresh leaves were used to extract total genomic DNA (gDNAs) with Plant Genomic DNA Kit (Tiangen Biotech Co., Beijing, China). The 350 bp paired end library was constructed according to the manufacturer’s introductions (Illumina, San Diego, CA) and sequenced on Illumina Hiseq 2500 platform (Illumina, San Diego, CA) for 150 bp paired-end sequencing. We obtained a data set containing a total of 32.6 G bp. To avoid reads with artificial bias (i.e. low quality paired reads, which primarily result from base calling duplicates and adaptor contamination), we removed the following types of reads: (i) reads with 3 nt unidentified nucleotides (N); (ii) reads aligned to the adaptor; (iii) reads with ≥20% bases having Phred quality ≥5. The complete chloroplast genome was assembled with GetOrganelle v1.7.2a (Jian et al. Citation2019; Luo et al., Citation2012), and the average read coverage was 4661× (range of read coverage: 29–7869×). GapCloser v2.04 (Luo et al. 2012) was used to fill gaps and MUMmer v3.23 (Kurtz et al. Citation2004) was used for alignment. The DOGMA tool (Dual Organellar GenoMe Annotator; http://dogma.ccbb.utexas.edu/) was used for annotation (Wyman et al. Citation2004). The complete H. salicifolia chloroplast genome was submitted to NCBI (https://www.ncbi.nlm.nih.gov, GenBank accession number: MW392804). Complete chloroplast genome sequences were aligned using MAFFT (version 7.452) (Katoh et al. Citation2019) and adjusted manually. Phylogenetic relationships among H. salicifolia and other Elaeagnaceae species were reconstructed with maximum likelihood using RAxML (Stamatakis Citation2014).
With this alignment of complete chloroplast genomes for several species of Hippophae and Elaeagnus, we found that, the gene content, average CG content and intron content of Hippophae are comparable to that of Elaeagnus (51.09% vs. 50.82%, 36.68% vs. 37.07%, 6.83% vs. 6.57%, respectively). However, we cannot find any genes or intergenic spacers that are particularly hyper-variable within Hippophae or Elaeagnus that could be used for species discrimination. The effective number of codons (ENC) of Hippophae and Elaeagnus chloroplast genomes coding sequence was 49.42–50.58 and 49.19–49.77, respectively, indicating a slightly weaker of codons bias in Elaeagnus.
Phylogenetic analysis recovered all seven Hippophae species as monophyletic with a clade containing H. salicifolia and H. gyantsensis as sister to the rest. All H. rhamnoides subspecies clustered in a well-supported clade (BP = 100%). Notably, H. neurocarpa nested into subclade H. rhamnoides (BP = 100%) (), which was inconsistent with the previous study (Jia and Bartish Citation2018). We speculate that the sample of the sequence NC_047483 does not belong to H. neurocarpa but to the hybrid of H. rhamnoides ssp. sinensis and H. neurocarpa, because the sample (NC_047483) was collected in the hybrid zone between H. rhamnoides ssp. sinensis and H. neurocarpa, and the hybrid individuals possess transitional morphology between their parents (Du et al. Citation2008). Incorrect identification or sampling error of H. neurocarpa (NC_047483) may led to this confusing result.
Disclosure statement
The authors report that they have no potential conflict of interest.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov under the accession no. MW392804. The raw sequence data used in this research were deposited successfully with registered numbers of associated BioProject, SRA, and Bio-Sample: PRJNA689712, SRR13357448, and SAMN17215125, respectively.
Additional information
Funding
References
- Du YJ, Wang YJ, Yang J, Zhang H, Sun K. 2008. Morphological analysis of Hippophae rhamnoides ssp. Sinensis×Hippophae neurocarpa ssp. neurocarpa hybrid zone. J Northwest Norm Univ (Nat Sci). 44(3):73–77.
- Jia DR, Bartish IV. 2018. Climatic changes and Orogeneses in the late Miocene of Eurasia: the main triggers of an expansion at a continental scale? Front Plant Sci. 9:1400.
- Jian JJ, Yu WB, Yang JB, Song Y, Li DZ. 2019. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21:241.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12.
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 1(1):18 doi:10.1186/2047-217X-1-18. PMC: 23587118
- Qin H, Gilbert MG. 2007. Elaeagnaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China, Vol. 13. Science Press & Missouri Botanical Garden Press, Beijing. 251–273.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Wang LY, Wang J, He CY, Zhang JG, Zeng YF. 2018. Whole chloroplast genome characterization and comparison of two sympatric species in genus Hippophae (Elaeagnaceae). J For Res. 100638.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Zhou W, Dong Q, Wang HL, Hu N. 2020. Characterization of the complete chloroplast genome of Hippophae tibetana. Mitochondrial DNA Part B. 5:593–594.
- Zhou W, Hu N, Dong Q, Wang H, Wang Y. 2019. Complete chloroplast genome sequences of Hippophae neurocarpa. Mitochondrial DNA Part B. 4(1):2048–2049.