Abstract
Sarcophila rasnitzyni Rohdendorf and Verves, 1985 (Diptera: Sarcophagidae) is of potential significance in medicine and epidemiology. In this study, we present the mitochondrial genome of S. rasnitzyni. The full length of the mitochondrial genome is 15,321 bp (GenBank accession no. MW592359), and 13 protein-coding genes (PCGs), two ribosomal RNAs (rRNAs), 22 transfer RNAs (tRNAs), and a non-coding control region were identified. Nucleotide composition is A 38.0%, G 9.9%, C 14.9%, T 37.2%, respectively. It reveals a strong A + T bias (75.2%). Phylogenetic analysis indicates that the species-level relationship between S. rasnitzyni and S. mongolica closely clusters together, and separates clearly from the rest of species. This study provides important genetic data for further enriching our understanding of phylogenetic relationship of sarcophagids species.
Sarcophagid flies (known as flesh flies) can lead to global health concerns as carriers of various pathogenic micro-organisms, additionally, they usually colonized on the decomposed corpses and constitute a part of the insect faunal succession representing important destruction stage responsible for the essential decomposition (Ren et al. Citation2018). Sarcophila rasnitzyni Rohdendorf and Verves, 1985 (Diptera: Sarcophagidae) mainly distributed in China (Beijing, Heilongjiang, Neimenggu, Qinghai, Xinjiang), Mongolia and Russia (East Siberia) (Pape Citation1996; Xu and Zhao Citation1996). The adult of S. rasnitzyni colonized on the decomposed carcasses (Xu and Zhao Citation1996). Except this species, only one species of Sarcophila genus (Sarcophila mongolica Chao and Zhang) has been recorded in China (Xu and Zhao Citation1996). Here, we present the complete mitochondrial genome (mitogenome) of S. rasnitzyni, which would further enrich our understanding of the phylogenetic relationship of Sarcophila genus (Cameron Citation2014).
In this study, the adult specimens of S. rasnitzyni were trapped by pig lung in June 2020, Beijing (40°22′N, 166°23′ E), China. All the specimens were identified by traditional morphological methods (Xu and Zhao Citation1996), and then soaked in 95% alcohol and placed in −20 °C (Xuzhou Medical University, Jiangsu, China) with a unique code (CSU20210201). Total DNA was extracted from thoracic muscle tissues of an adult specimen using QIANamp Micro DNA Kit (Qiangen Biotech Co., Ltd, Germany). The mitogenome sequencing of S. rasnitzyni was performed on an Illumina HiSeq 2500 Platform (pared-end 150 bp), and then assembled based on Illumina short reads with MitoZ v2.3 (Meng et al. Citation2019). The rough boundaries of all genes was annotated by MITOS2 Web Server (http://mitos2.bioinf.uni-leipzig.de/index.py) under the invertebrate mitochondrial code (Bernt et al. Citation2013). In order to confirm the correctness of gene boundaries, 13 protein coding genes (PCGs) were aligned with published mitogenomes of flesh flies using Muscle (codons) as implemented in MEGA X (Kumar et al. Citation2018). Two ribosomal RNAs (rRNAs) were aligned by the Q-INSi method as implemented in MAFFT v7.263 (Katoh and Standley Citation2016). 22 transfer RNAs (tRNAs) were predicted by tRNAscan-SE Search Server v1.21 (Lowe and Chan Citation2016) and verified by aligning with other dipteran insects.
In this study, the total length of the mitogenome of S. rasnitzyni is 15,321 bp (GenBank accession no. MW592359). It obtains 13 PCGs, two rRNAs, 22 tRNAs, and a non-coding control region. The gene arrangement is consistent with that of ancestral metazoan (Cameron 2014). All PCGs initiate with a typical start codon (ATN), except that cox1 starts with TCG. Most PCGs end with the termination codons TAA/TAG, in addition that four genes (cox1, cox2, nad4 and nad5) are terminated with T. Nucleotide composition of S. rasnitzyni is A 38.0%, G 9.9%, C 14.9%, T 37.2%. It indicates a highly A + T bias (75.2%). Additionally, the size of overlap regions was examined, varying from 1 to 9 bp. The longest intergenic spacers (18 bp) are situated between trnE and trnF.
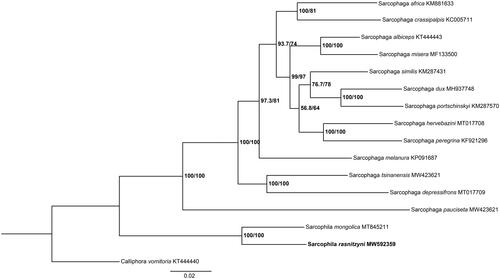
Furthermore, phylogenetic tree was constructed with S. rasnitzyni and other 14 sarcophagids species based on the 13 PCGs by maximum likelihood (ML) method with the GTR + G + I model as implemented in IQ-TREE v.1.6.12 (Nguyen et al. Citation2015). Calliphora vomitoria (Diptera: Calliphoridae) was used as an outgroup (). In the phylogenetic tree, the species-level relationship between S. rasnitzyni and S. mongolica closely clusters together, and separates clearly from the rest of species. The branches are well supported. This study provides important mitochondrial data for further enriching our understanding of phylogenetic relationship of sarcophagids species.
Acknowledgments
We are grateful to Prof. Lushi Chen (Guizhou Police College) for species identification.
Disclosure statement
The authors have declared that no competing interests exist.
Data availability statement
Mitogenome data supporting this study are openly available in GenBank at: https://www.ncbi.nlm.nih.gov/nuccore/MW592359. Associated BioProject, SRA, and BioSample accession numbers are https://dataview.ncbi.nlm.nih.gov/object/PRJNA700699, https://www.ncbi.nlm.nih.gov/sra/SRR13757193, and SAMN17838720, respectively.
Additional information
Funding
References
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Cameron SL. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu Rev Entomol. 59:95–117.
- Katoh K, Standley DM. 2016. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics. 32(13):1933–1942.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Meng GL, Li YY, Yang CT, Liu SL. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Pape T. 1996. Catalogue of the Sarcophagidae of the World (Insecta: Diptera). Memoirs of Entomology International. 8:1–558.
- Ren L, Shang Y, Chen W, Meng F, Cai J, Zhu G, Chen L, Wang Y, Deng J, Guo Y. 2018. A brief review of forensically important flesh flies (Diptera: Sarcophagidae). Forensic Sci Res. 3(1):16–26.
- Xu WQ, Zhao JM. 1996. Flies of China. Shenyang: Liaoning Science and Technology.