Abstract
Leucoagaricus naucinus (Fr.) Singer is a mycorrhizal fungus widely distributed in the northern Hemisphere. In the present study, the complete mitochondrial genome of Leucoagaricus naucinus was sequenced, assembled, and annotated. The L. naucinus mitochondrial genome was composed of circular DNA molecules, with the total size of 61,434 bp. The GC content of the L. naucinus mitochondrial genome was 26.07%. A total of 30 protein-coding genes (PCGs), two ribosomal RNA (rRNA) genes, and 26 transfer RNA (tRNA) genes were detected in the L. naucinus mitochondrial genome. Phylogenetic analysis based on combined mitochondrial gene dataset indicated that the L. naucinus exhibited a close relationship with Agaricus bisporus.
Leucoagaricus naucinus (Fr.) Singer is called Smooth Lepiota. This species is widely distributed in the northern Hemisphere, especially in China and North America (Vellinga et al. Citation2003), whose fruit bodies occur in autumn and can be found in spring and summer as well. Although this species is very common, the information of genetic characteristics of the species is very limited (Ning et al. Citation2016). It is recognized by its white cap, white gills, and white ring. The phenolic composition of the L. naucinus exhibited strong antimicrobial activity against some foodborne and spoilage bacteria (Aslim and Ozturk Citation2011). Up to now, the mitochondrial genome of Leucoagaricus genus has not been revealed. Mitochondrial genome has been widely used to analyze the phylogeny, evolution, and classification of fungal species (Zhang et al. Citation2017; Li et al. Citation2019b, Citation2020a). This study served as the first report on the complete mitochondrial genome from the genus Leucoagaricus, which will promote the understanding of phylogeny, evolution, and taxonomy of this important fungal species.
The specimen (L. naucinus) was collected from Sichuan, China (101.48 E; 27.41 N). The specimen was identified as L. naucinus by morphology, internal transcribed spacer (ITS) sequence and small subunit ribosomal RNA (rRNA) (rns) sequence. A specimen was deposited in collection center of Chengdu University (Qiang Li, [email protected]) under the voucher number of s2148. We sequenced and de novo assembled the complete mitochondrial genome of L. naucinus according to previous described methods (Li et al. Citation2019a, Citation2019b; Wang et al. Citation2020a, Citation2020b). Briefly, the complete mitochondrial genome of L. naucinus was assembled using NOVOPlasty v4.3.1 (Dierckxsens et al. Citation2017; Li et al. Citation2020b), which was circularly assembled at the K-mer size of 27. We then annotated the protein-coding genes (PCGs), rRNA genes, tRNA genes, and introns of the L. naucinus mitochondrial genome using MITOS (Bernt et al. Citation2013) and MFannot (Valach et al. Citation2014), both based on the genetic code 4. PCGs or ORFs in the L. naucinus mitochondrial genome were also predicted based on the NCBI Open Reading Frame (ORF) Finder (NCBI Resource Coordinators Citation2018), and then annotated by BLASTP searches against the NCBI non-redundant protein sequence database (Bleasby and Wootton Citation1990). The tRNA genes in the L. naucinus mitochondrial genome were also predicted with tRNAscan-SE v1.3.1 (Lowe and Chan Citation2016).
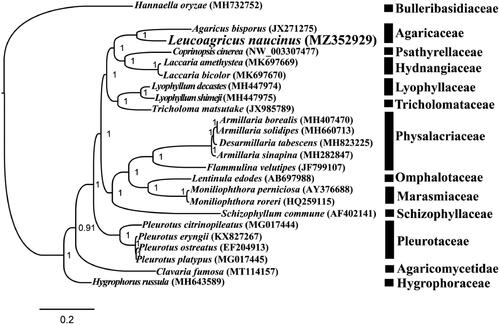
The complete mitochondrial genome of L. naucinus is 61,434 bp in length. The base composition of the L. naucinus mitochondrial genome is as follows: A (37.82%), T (36.11%), G (13.56%), and C (12.51%). A total of 30 PCGs, two rRNA genes (rns and rnl), and 26 transfer RNA (tRNA) genes were detected in the L. naucinus mitochondrial genome. The complete mitochondrial genome of L. naucinus contained 18 introns, 14 of which were located in PCGs, including cox1, cob, and nad5. All of these introns were belonged to the group I, which were named according to previously described method (Zhang and Zhang Citation2019). To reveal the phylogenetic relationships of Agaricales species, we constructed a phylogenetic tree for 23 Agaricales species. Hannaella oryzae from the order Tremellales was set as outgroup (Li et al. Citation2021a). Bayesian analysis (BI) method was used to construct the phylogenetic tree based on the combined 14 core PCGs (Cheng et al. Citation2021; Li et al. Citation2021b, Citation2020c). Single mitochondrial genes were first aligned using MAFFT v7.037 (Katoh et al. Citation2019), and then concatenated into a gene dataset using the SequenceMatrix v1.7.8 (Vaidya et al. Citation2011). Best-fit models of evolution and partitioning schemes were detected using PartitionFinder 2.1.1 (Lanfear et al. Citation2017). We analyze the phylogenetic relationships of the 23 Agaricales species using MrBayes v3.2.6 (Ronquist et al. Citation2012) based on the combined gene dataset. As shown in the phylogenetic tree (), the mitochondrial genome of L. naucinus exhibited a close relationship with Agaricus bisporus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. MZ352929. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA734961, SRR14737568, and SAMN19551202, respectively.
References
- Aslim B, Ozturk S. 2011. Phenolic composition and antimicrobial and antioxidant activities of Leucoagaricus leucothites (Vittad.) Wasser. J Med Food. 14(11):1419–1424.
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Bleasby AJ, Wootton JC. 1990. Construction of validated, non-redundant composite protein sequence databases. Protein Eng. 3(3):153–159.
- Cheng J, Luo Q, Ren YH, Luo Z, Liao WL, Wang X, Li Q. 2021. Panorama of intron dynamics and gene rearrangements in the phylum Basidiomycota as revealed by the complete mitochondrial genome of Turbinellus floccosus. Appl Microbiol Biotechnol. 105(5):2017–2032.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34(3):772–773.
- Li Q, He X, Ren Y, Xiong C, Jin X, Peng L, Huang W. 2020a. Comparative mitogenome analysis reveals mitochondrial genome differentiation in ectomycorrhizal and asymbiotic Amanita species. Front Microbiol. 11:1382.
- Li Q, Li L, Feng H, Tu W, Bao Z, Xiong C, Wang X, Qing Y, Huang W. 2021a. Characterization of the complete mitochondrial genome of Basidiomycete yeast Hannaella oryzae: intron evolution, gene rearrangement, and its phylogeny. Front Microbiol. 12:646567.
- Li Q, Ren Y, Shi X, Peng L, Zhao J, Song Y, Zhao G. 2019a. Comparative mitochondrial genome analysis of two ectomycorrhizal fungi (Rhizopogon) reveals dynamic changes of intron and phylogenetic relationships of the subphylum Agaricomycotina. Int J Mol Sci. 20(20):5167.
- Li Q, Ren Y, Xiang D, Shi X, Zhao J, Peng L, Zhao G. 2020b. Comparative mitogenome analysis of two ectomycorrhizal fungi (Paxillus) reveals gene rearrangement, intron dynamics, and phylogeny of basidiomycetes. IMA Fungus. 11(1):12.
- Li Q, Wu P, Li L, Feng H, Tu W, Bao Z, Xiong C, Gui M, Huang W. 2021b. The first eleven mitochondrial genomes from the ectomycorrhizal fungal genus (Boletus) reveal intron loss and gene rearrangement. Int J Biol Macromol. 172:560–572.
- Li Q, Xiang D, Wan Y, Wu Q, Wu X, Ma C, Song Y, Zhao G, Huang W. 2019b. The complete mitochondrial genomes of five important medicinal Ganoderma species: features, evolution, and phylogeny. Int J Biol Macromol. 139:397–408.
- Li Q, Yang L, Xiang D, Wan Y, Wu Q, Huang W, Zhao G. 2020c. The complete mitochondrial genomes of two model ectomycorrhizal fungi (Laccaria): features, intron dynamics and phylogenetic implications. Int J Biol Macromol. 145:974–984.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- NCBI Resource Coordinators. 2018. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 46(D1):D8–D13.
- Ning YJ, Wang SS, Chen QJ, Ling ZR, Wang SN, Wang WP, Zhang GQ, Zhu MJ. 2016. An extracellular yellow laccase with potent dye decolorizing ability from the fungus Leucoagaricus naucinus LAC-04. Int J Biol Macromol. 93(Pt A):837–842.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Vaidya G, Lohman DL, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 27(2):171–180.
- Valach M, Burger G, Gray MW, Lang BF. 2014. Widespread occurrence of organelle genome-encoded 5S rRNAs including permuted molecules. Nucleic Acids Res. 42(22):13764–13777.
- Vellinga EC, de Kok RP, Bruns TD. 2003. Phylogeny and taxonomy of Macrolepiota (Agaricaceae). Mycologia. 95(3):442–456.
- Wang X, Song A, Wang F, Chen M, Li X, Li Q, Liu N. 2020a. The 206 kbp mitochondrial genome of Phanerochaete carnosa reveals dynamics of introns, accumulation of repeat sequences and plasmid-derived genes. Int J Biol Macromol. 162:209–219.
- Wang X, Wang YJ, Yao W, Shen JW, Chen MY, Gao M, Ren JN, Li Q, Liu N. 2020b. The 256 kb mitochondrial genome of Clavaria fumosa is the largest among phylum Basidiomycota and is rich in introns and intronic ORFs. IMA Fungus. 11(1):26.
- Zhang S, Zhang YJ. 2019. Proposal of a new nomenclature for introns in protein-coding genes in fungal mitogenomes. IMA Fungus. 10(15):15.
- Zhang YJ, Zhang HY, Liu XZ, Zhang S. 2017. Mitochondrial genome of the nematode endoparasitic fungus Hirsutella vermicola reveals a high level of synteny in the family Ophiocordycipitaceae. Appl Microbiol Biotechnol. 101(8):3295–3304.