Abstract
Here, we report the complete mitogenome information of the six-line wrasse Pseudocheilinus hexataenia (Bleeker, 1857). Genome sequencing using the Illumina HiSeq platform allowed the assembly of a circular mitochondrial genome of 17,111 bp from P. hexataenia, consisting of 54% AT nucleotides, 13 protein-coding genes (PCGs), two ribosomal RNA (rRNA) genes, 22 transfer RNA (tRNA) genes, and a putative control region in the typical Labriformes gene composition. The gene order of the P. hexataenia mitochondrion was identical to that of the Labridae mitogenomes. Phylogenetic reconstruction places P. hexataenia with a close relationship with the mitogenome of the goldsinny wrasse, Ctenolabrus rupestris.
The family Labridae, one of the most conspicuous polyphyletic marine fish, has over 630 species in 88 genera (Nelson et al. Citation2016) and shows high species richness, abundance, and structural and ecological diversity, resulting in one of the top five families in coral reefs (Bellwood Citation2019). Labrids include a large group of both wrasses and parrotfish, crucial in many coral reef ecosystems. In recent decades, significant progress has been made in resolving the phylogenetic relationships among the complex lineages of both tropical and temperate labrids (Westneat and Alfaro Citation2005; Cowman et al. Citation2009; Price et al. Citation2011; Phillips et al. Citation2016; Baliga and Law Citation2016). The six-line wrasse Pseudocheilinus hexataenia (Bleeker 1857) is widely distributed in the tropical marine waters of the Indo-Pacific. The six-line wrasse is a popular ornamental species because of its small size (∼10 cm), high activity in aquaria, and unique body color, recognized by the six distinct horizontal orange stripes along the violet flanks. In general, cleaner wrasses play a fundamental ecological role in interspecific symbiosis by removing parasites and infected tissues from the surfaces of fish clients. The six-line wrasse is a facultative cleaner, as it may occasionally clean, primarily at the juvenile stage. Although many whole mitogenomes have been published in the Labridae, there is no information on the complete mitogenome in Pseudocheilines. Therefore, the objective of this report is to bring together the phylogenetic information on the genus Pseudocheilinus as a basis for further evaluation of Labridae evolution.
A specimen of P. hexataenia was collected from the East China Sea (30°45 N 126°22'E) in July 2013. The specimen and DNA were deposited at the Research Institute of Basic Sciences of Incheon National University (Specimen ID: 2013- Labridae-01; https://www.inu.ac.kr/user/indexMain.do?siteId=ribs; Sang-Eun Nam; [email protected]) (Nam and Rhee Citation2020). All animal handling and experimental procedures were approved by the Animal Welfare Ethical Committee and the Animal Experimental Ethics Committee of the Incheon National University (Incheon, South Korea). Genomic DNA was prepared from a specimen muscle using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). A fragment library was prepared using the TruSeq DNA Sample Preparation Kit (Illumina, San Diego, CA, USA). The sequencing library was prepared by random fragmentation of the DNA sample, followed by 5′ and 3′ adapter ligation. Raw reads were obtained from the sample that passed the quality control check on the Illumina HiSeq platform (Illumina) at Macrogen, Inc. (Seoul, South Korea). Adapter sequences, low-quality reads, reads with > 10% of unknown bases, and ambiguous bases were removed from FASTAQ with Trimmomatic ver. 0.40 (Illumina) using default trimming steps. After the quality check process, 35,698,204 filtered reads were obtained from 45,836,020 raw reads. Subsequently, de novo assembly was conducted with various k-mers using SPAdes ver. 3.12.0 (Bankevich et al. Citation2012) with default settings and the “isolate” option enabled. The resulting circular contig consensus sequence was annotated using MITOS2 (Bernt et al. Citation2013) and tRNAscan-SE 2.0 (Lowe and Eddy Citation1997). BLAST searches confirmed the identity of the genes (http://blast.ncbi.nlm.nih.gov).
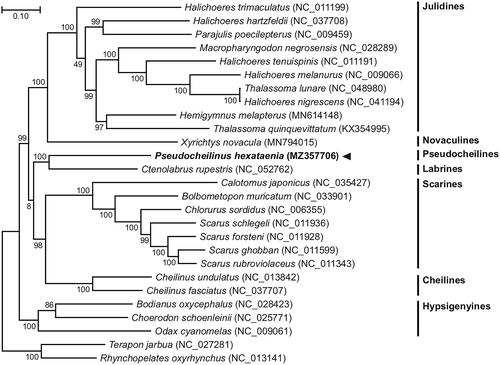
The P. hexataenia circular 17,111 bp mitogenome (GenBank accession no. MZ357706) was composed of the following nucleotide composition: 27.3% A, 29.0% C, 17.6% G, and 26.2% T. The gene order and composition of the P. hexataenia mitogenome are identical to those of all known wrasse mitogenomes. Thirteen P. hexataenia mitochondrial PCGs begin with an ATG start codon, and the 22 tRNAs have typical cloverleaf secondary structures. We reconstructed a phylogenetic tree using the concatenated set of all 13 PCGs of the P. hexataenia mitogenome, 24 published complete mitogenomes belonging to Labriformes, and two Centrarchiformes species as an outgroup (). JModelTest ver. 2.1.10 (Darriba et al. Citation2012) was used to select the best substitution model, and a substitution model (HKY + G + I) was applied to perform a maximum-likelihood (ML) method in PhyML ver. 2.4.5 (Guindon and Gascuel Citation2003) with 1000 bootstrap replicates. The P. hexataenia mitogenome formed a sister group with the mitogenome of the goldsinny wrasse, C. rupestris, with strong support. The overall topology was consistent with previous phylogenetic results (Phillips et al. Citation2016). Our results also agree that Pseudocheilines, including Pseudocheilinus, were not the sister-group to the Cheilines and were split off from the Cheilines and placed at the next node up the tree (Westneat and Alfaro Citation2005). Additional whole mitogenomes of Pseudocheilines and Labrines are needed for a more detailed analysis of their phylogenetic relationships.
Figure 1. Maximum-likelihood (ML) phylogeny of 24 published complete mitogenomes belonging to Labriformes and two Centrarchiformes species as an outgroup based on the concatenated nucleotide sequences of protein-coding genes (PCGs). The phylogenetic analysis was performed using the maximum likelihood method, GTR + G + I model with a bootstrap of 1000 replicates. Numbers on the branches indicate ML bootstrap percentages. DDBJ/EMBL/Genbank accession numbers for published sequences are incorporated. The black triangle indicates the fish analyzed in this study.
Author’ contributions
S.-E. Nam: Conceptualization, Methodology, Software, Writing
H.-J. Eom: Methodology, Software, Data curation
H.S. Park: Conceptualization, Visualization, Reviewing and Editing
J.-S. Rhee: Conceptualization, Supervision, Reviewing and Editing
Disclosure statement
The authors report no conflicts of interest and are solely responsible for the content and writing of this manuscript.
Data availability statement
BioProject, BioSample, and SRA accession numbers are https://www.ncbi.nlm.ni h.gov/bioproject/PRJNA743750, https://www.ncbi.nlm.nih.gov/biosample/SAMN20059971, and https://www.ncbi.nlm.nih.gov/sra/?term=SRR15348071, respectively. The data that support the findings of this study are available at the National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov, with the accession number MZ357706.
Additional information
Funding
References
- Baliga VB, Law CJ. 2016. Cleaners among wrasses: phylogenetics and evolutionary patterns of cleaning behavior within Labridae. Mol Phylogenet Evol. 94(Pt A):424–435.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Bellwood DR, Schultz O, Siqueira AC, Cowman PF. 2019. A review of the fossil record of the Labridae. Annalen Des Naturhistorischen Museums in Wien, Serie A. 121:125–193.
- Bernt A, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Cowman PF, Bellwood DR, van Herwerden L. 2009. Dating the evolutionary origins of wrasse lineages (Labridae) and the rise of trophic novelty on coral reefs. Mol Phylogenet Evol. 52(3):621–631.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772.
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 52(5):696–704.
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25(5):955–964.
- Nam S-E, Rhee J-S. 2020. Complete mitochondrial genome of the lemon damsel, Pomacentrus moluccensis (Perciformes, Pomacentridae). Mitochondrial DNA B Resour. 5(3):2157–2158.
- Nelson JS, Grande TC, Wilson MVH. 2016. Fishes of the world. 5th ed. New Jersey: John Wiley and Sons.
- Phillips GA, Carleton KL, Marshall NJ. 2016. Multiple genetic mechanisms contribute to visual sensitivity variation in the Labridae. Mol Biol Evol. 33(1):201–215.
- Price SA, Holzman R, Near TJ, Wainwright PC. 2011. Coral reefs promote the evolution of morphological diversity and ecological novelty in labrid fishes. Ecol Lett. 14(5):462–469.
- Westneat MW, Alfaro ME. 2005. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol Phylogenet Evol. 36(2):370–390.
