Abstract
Amorpha californica var. napensis Jeps. 1925, the Napa false indigo, is a threatened shrub endemic to northern California. Here the complete chloroplast genome of topotype material of var. napensis was assembled and characterized to contribute to the bioinformatics, systematics, and conservation of this variety. The chloroplast genome (GenBank accession OK274088) is 158,294 base pairs (bp) in length, encodes 130 genes including 85 protein-coding, 37 tRNA, 8 rRNA, and shows a high-level of gene synteny to other Papilionoideae. Phylogenetic analysis fully resolved var. napensis in a clade with A. fruticosa L. and A. roemeriana Scheele, sister to the Dalbergieae. The newly sequenced chloroplast genome shows that the genetic differences between var. napensis and Amorpha californica Nutt. var. californica are greater than the variation observed between var. napensis and many other Amorpha spp. sequences deposited in GenBank. These data suggest that var. napensis should be elevated to full species rank.
Amorpha californica var. napensis is a deciduous shrub originally described by W.L. Jepson from specimens collected from Moore Creek, Howell Mountain, Napa County, California (Jepson Citation1925). The variety was said to differ from A. californica Nutt. var. californica in being subglabrous (vs. minutely pubescent), lacking glands on the rachis (vs. prickle-like glands), having shorter racemes 2.5–3.18 cm in length (vs. 5–14 cm), and displaying minute teeth on the calyx (vs. long teeth) (Jepson Citation1925). Abrams (Citation1944) considered var. napensis a form of A. californica var. hispidula (Greene) E.J.Palmer, whereas Munz (Citation1959) treated the latter as a synonym of var. napensis. Wilbur (Citation1975) accepted the varietal status of var. napensis, but placed var. hispidula in synonymy under A. californica. Hickman (Citation1993) and Baldwin et al. (Citation2012) recognized the variety, but narrowed its distribution to Napa, Marin, and Sonoma counties where it occurs in Chaparral communities less than 800 meters in elevation. Calflora (https://www.calflora.org/) currently lists var. napensis as 1B.2 (fairly threatened in California). Based on a survey of this variety from the type locality, it appears nearly extirpated due to vineyard expansion in the famed Howell Mountain American Viticultural Area. To date, the only data deposited in GenBank for var. napensis are three barcode sequences determined from a single specimen (Straub and Doyle Citation2014). We assembled and analyzed the complete plastid genome of topotype material of var. napensis to contribute to the bioinformatics, systematics, and conservation efforts of this threatened variety.
The specimen of var. napensis analyzed in this study was collected in accordance with guidelines provided by Napa county and Hartnell College from the north end of Moore Creek, Angwin, California (38°33′55.0152″N 122°24′18.8028″W) and deposited in the herbarium at Hartnell College (https://www.hartnell.edu/, Jeffery R. Hughey, [email protected]) under voucher number HCC 266. The DNA was extracted following the methods outlined in Hughey et al. (Citation2019). The 150 bp PE Illumina library construction and sequencing was performed by Quick Biology (Pasadena, California, USA) and yielded 24,733,870 reads. The adapters and low quality reads were removed using the Trim Adapters and Trim Low Quality default settings with the BBDuk plugin in Geneious Prime version 2019.1.3 (Biomatters Limited, Auckland, New Zealand). The genome was assembled by mapping the reads onto the reference genome A. fruticosa, GenBank accession number MN709789 (Zhang, Wang, et al. Citation2020), using the Medium-Low Sensitivity/Fast setting in Geneious Prime. The gaps were closed by iterative mapping using the same settings in Geneious Prime. The annotation was performed using the default settings in GeSeq (Tillich et al. Citation2017) and CPGAVAS2 (Shi et al. Citation2019), followed by adjustments according to NCBI ORFfinder, Sequin 15.5, and tRNAscan-SE 1.21 (Schattner et al. Citation2005). The var. napensis complete chloroplast nucleotide sequence was aligned to 25 other papilionoid and three outgroup taxa from the Dialioideae using the auto settings in MAFFT (Katoh and Standley Citation2013). The ML phylogenetic analysis was executed with the TVM + F + I + G4 substitution model and 1000 ultrafast bootstrap replicates in W-IQ-TREE (Trifinopoulos et al. Citation2016). The tree was visualized with TreeDyn 198.3 at Phylogeny.fr (Dereeper et al. Citation2008).
The complete chloroplast genome of var. napensis is 158,294 bp in length and exhibits a standard quadripartite structure (Shinozaki et al. Citation1986; Wicke et al. Citation2011). The genome contains an LSC, SSC, and two IRs with lengths 88,110 bp, 18,580 bp and 25,802 bp, respectively. The GC content is 36.0%. Gene content and organization show a high-level of synteny to A. fruticosa and A. roemeriana (GenBank accession number MW628937) (Zhang, Wang, et al. Citation2020; Lee et al. Citation2021). The chloroplast genome of var. napensis is 99.86% similar in nucleotide sequence to A. fruticosa and 99.87% to A. roemeriana. BLAST analysis of the var. napensis genome found an identical petN-psbM intergenic spacer sequence and nearly identical trnT-trnD intergenic spacer sequence that differed by 1 bp from var. napensis from Angwin, California (Straub and Doyle Citation2014). In comparison, var. napensis differed from var. californica from the Santa Rosa Mountains, California in its petN-psbM sequence by 5 bp (99.0%) and trnT-trnD sequence by 12 bp (99.24%). The var. napensis sequences were greater in genetic distance to var. californica than to many other species classified in Amorpha.
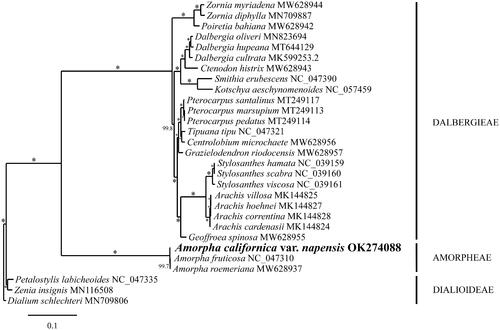
Phylogenetic analysis of representative Papilionoideae fully resolved var. napensis in a clade with A. fruticosa and A. roemeriana in a sister position to the Dalbergieae (). These results are consistent with the evolutionary relationships inferred for the Amorpheae based on multigene analysis (McMahon and Hufford Citation2004), matK sequencing (Cardoso et al. Citation2012, Citation2013), and a schematic compilation based on other published works (The Legume Phylogeny Working Group Citation2013). Given the high degree of plastid marker sequence variation between var. napensis and var. californica, phylogenetic analysis of barcode markers or the chloroplast genome of var. californica from its type locality in Santa Barbara, California, are necessary to test the hypothesis that var. napensis should be recognized at the species level.
Figure 1. RaxML phylogram of the complete chloroplast genome of Amorpha californica var. napensis and related Papilionoideae. Numbers along the branches are ML bootstrap supports based on 1000 replicates (* indicates 100% boostrap support). The legend below represents the scale for nucleotide substitutions. The following accessions with references were used for the phylogenetic anlaysis: Amorpha roemeriana MW628937, Centrolobium microchaete MW628956, Ctenodon histrix MW628943, Geoffroea spinosa MW628955, Grazielodendron riodocensis MW628957, Poiretia bahiana MW628942, Zornia myriadena MW628944 (Lee et al. Citation2021); Amorpha fruticosa NC_047310, Dialium schlechteri MN709806, Petalostylis labicheoides NC_047335, Smithia erubescens NC_047390, Tipuana tipu NC_047321, Zornia diphylla MN709887 (Zhang, Wang, et al. Citation2020); Pterocarpus marsupium MT249113, Pterocarpus pedatus MT249114, Pterocarpus santalinus MT249117 (Hong et al. Citation2020); Stylosanthes hamata NC_039159, Stylosanthes scabra NC_039160, Stylosanthes viscosa NC_039161 (Marques et al. Citation2018); Arachis cardenasii MK144824, Arachis hoehnei MK144827, Arachis villosa MK144825 (Wang et al. Citation2019); Dalbergia hupeana MT644129 (Hong et al. Citation2021); Dalbergia oliveri MN823694 (Zhang, Li, et al. Citation2020); Kotschya aeschynomenoides NC_057459 (Oyebanji et al. Citation2020); Dalbergia cultrata MK599253 (Liu et al. Citation2019); Zenia insignis MN116508 (Lai et al. Citation2019); Amorpha californica var. napensis OK274088 (this study).
Author contributions
All authors were equally involved in the analysis and interpretation of the data; the drafting of the paper; revising it critically for intellectual content; and the final approval of the version to be published. The corresponding author Jeffery R. Hughey and coauthor Richard A. Stabler jointly conceived and designed the project. All authors agreed to be accountable for all aspects of this work.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank at (https://www.ncbi.nlm.nih.gov/) under the accession number OK274088. The associated BioProject, SRA, and BioSample numbers are PRJNA765780, SRR16037173, and SAMN21583931 respectively.
Additional information
Funding
References
- Abrams L. 1944. Illustrated flora of the Pacific states: Washington, Oregon, and California. Vol. 2. Polygonaceae to Krameriaceae. Stanford (CA): Stanford University Press.
- Baldwin BG, Goldman DH, Keil DJ, Patterson R, Rosatti TJ, Wilken DH, editors. 2012. The Jepson manual: vascular plants of California. 2nd ed. Berkeley (CA): University of California Press.
- Cardoso D, de Queiroz LP, Pennington RT, de Lima HC, Fonty E, Wojciechowski MF, Lavin M. 2012. Revisiting the phylogeny of Papilionoid legumes: new insights from comprehensively sampled early-branching lineages. Am J Bot. 99(12):1991–2013.
- Cardoso D, Pennington RT, de Queiroz LP, Boatwright JS, Van Wyk BE, Wojciechowski MF, Lavin M. 2013. Reconstructing the deep-branching relationships of the Papilionoid legumes. S Afr J Bot. 89:58–75.
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36(Web Server issue):W465–W469.
- Hickman JC. 1993. The Jepson manual: higher plants of California. Berkeley (CA): University of California Press.
- Hong Z, Peng D, He W, Zhang N, Yang Z, Tembrock LR, Wu Z, Liao X, Xu D. 2021. Comparative analyses of 35 complete chloroplast genomes from the genus Dalbergia (Fabacaeae) and the identification of DNA barcodes for tracking illegal logging and counterfeit rosewood. Int J Mol Sci. 21(11):3758 (1-18).
- Hong Z, Wu Z, Zhao K, Yang Z, Zhang N, Guo J, Tembrock LR, Xu D. 2020. Comparative analyses of five complete chloroplast genomes from the genus Pterocarpus (Fabacaeae). IJMS. 21(11):3758.
- Hughey JR, Maggs CA, Mineur F, Jarvis C, Miller KA, Shabaka SH, Gabrielson PW. 2019. Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies). J Phycol. 55(3):503–508.
- Jepson WL. 1925. A manual of the flowering plants of California. Berkeley (CA): Associated students store, University of California, Berkeley.
- Katoh K, Standley DM. 2013. MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Lai Q, Tu T, Zhang D. 2019. The complete plastid genome of Zenia insignis Chun (Leguminosae). Mitochondrial DNA B Resour. 4(2):2926–2927.
- Lee C, Choi IS, Cardoso D, de Lima HC, de Queiroz LP, Wojciechowski MF, Jansen RK, Ruhlman TA. 2021. The chicken or the egg? Plastome evolution and an independent loss of the inverted repeat in papilionoid legumes. Plant J. 107(3):861–875.
- Liu Y, Huang P, Li CH, Zang FQ, Zheng YQ. 2019. Characterization of the complete chloroplast genome of Dalbergia cultrata (Leguminosae). Mitochondrial DNA B Resour. 4(2):2369–2370.
- Marques A, Moraes L, Aparecida Dos Santos M, Costa I, Costa L, Nunes T, Melo N, Simon MF, Leitch AR, Almeida C, et al. 2018. Origin and parental genome characterization of the allotetraploid Stylosanthes scabra Vogel (Papilionoideae, Leguminosae), an important legume pasture crop. Ann Bot. 122(7):1143–1159.
- McMahon M, Hufford L. 2004. Phylogeny of Amorpheae (Fabaceae: Papilionoideae). Am J Bot. 91(8):1219–1230.
- Munz PA. 1959. A California flora. Berkeley (CA): University of California Press.
- Oyebanji O, Zhang R, Chen SY, Yi TS. 2020. New insights into the plastome evolution of the Millettioid/Phaseoloid clade (Papilionoideae, Leguminosae). Front Plant Sci. 11:151.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:686–689.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73.
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, et al. 1986. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. Embo J. 5(9):2043–2049.
- Straub SC, Doyle JJ. 2014. Molecular phylogenetics of Amorpha (Fabaceae): an evaluation of monophyly, species relationships, and polyploid origins. Mol Phylogenet Evol. 76:49–66.
- The Legume Phylogeny Working Group, Bruneau A, Doyle JJ, Herendeen P, Hughes C, Kenicer G, Lewis G, Mackinder B, Pennington RT, Sanderson MJ, Wojciechowski MF, et al. 2013. Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon. 62:217–248.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235.
- Wang J, Li Y, Li C, Yan C, Zhao X, Yuan C, Sun Q, Shi C, Shan S. 2019. Twelve complete chloroplast genomes of wild peanuts: great genetic resources and a better understanding of Arachis phylogeny. BMC Plant Biol. 19(1):504.
- Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. 2011. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 76(3-5):273–297.
- Wilbur RL. 1975. A revision of the North American genus Amorpha (Leguminosae-Psoraleae). Rhodora. 77:337–409.
- Zhang J, Li Y, Heng S, Wang Y. 2020. The complete chloroplast genome sequence of Dalbergia oliveri. Mitochondrial DNA B Resour. 5(1):707–708.
- Zhang R, Wang YH, Jin JJ, Stull GW, Bruneau A, Cardoso D, De Queiroz LP, Moore MJ, Zhang SD, Chen SY, et al. 2020. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Syst Biol. 69(4):613–622.
