Abstract
Polydora hoplura Claparede 1868 (Polychaeta, Spionidae) is a mollusk shell-boring species which has been reported as a harmful species. In the present study, we sequenced and reported the mitochondrial genome (mitogenome) of P. hoplura from Korea. Its mitogenome was determined by long range PCR and primer walking. The mitogenome was found to be 17,707 bp in length and contained two ribosomal RNAs (rRNAs), 23 transfer RNAs (tRNAs), and 12 protein-coding genes which showed different gene arrangement with that in related mitogenomes of species of Sedentaria (Annelida). Interestingly, the atp8 gene was not found in the mitogenome of P. hoplura, whereas it is present in the mitogenomes of related species P. brevipalpa and P. websteri. Moreover, nad2 (3’ end) and cox1 (5’ end) genes overlapped in 23 bases. The mitogenome structure and gene contents of P. hoplura provide useful information on the evolution and phylogenetic relationship among polychaete species.
Species belonging to the genus Polydora (Spionidae, Polychaeta) have been reported as harmful mollusk shell-boring species (Radashevsky and Migotto Citation2017; Sato-Okoshi et al. Citation2017). In abalone aquaculture in Korea, Japan, and China, production has been seriously affected by shell-boring Polydora species (Radashevsky and Migotto Citation2017; Sato-Okoshi et al. Citation2013; Won et al. Citation2013). Therefore, accurate identification of spionid polychaetes using genetics is essential for monitoring shell-boring Polydora species. In our previous study, we identified two Korean Polydora species (P. haswelli and P. hoplura) by molecular phylogenetic analyses using a taxon-specific molecular marker (Lee et al. Citation2020).
A sample of P. hoplura analyzed in our previous molecular phylogenetic study was used for complete mitochondrial genome (mitogenome) sequencing (Lee et al. Citation2020; the sample was obtained from an aquaculture farm in Wando, Korea, in December 2015; 34°18′14.9″N, 126°46′14.7″E). The voucher specimen and the extracted total genomic DNA have been deposited in the Mokpo Regional Office, National Fishery Products Quality Management Service (NFQS) [the voucher number (NFQS-Polydora-2015-N001), contact person (Soon Jeong Lee, [email protected])]. Total genomic DNA was extracted from the body tissues of Polydora isolate from an abalone using DNeasy Blood & Tissue Kit (Qiagen, Germany). PCR products covering the complete mitogenome of P. hoplura were acquired using long range PCR. The complete mitogenome sequence was determined by primer walking for PCR products (Macrogen, Seoul, Korea). All Sanger sequencing data were finally assembled using Sequencer 5.4.6 (Gen Code, Ann Arbor, MI, USA) and Geneious Prime (https://www.geneious.com). Gene structure mapping and gene annotation were conducted using the BLAST search and ORF finder in GenBank (NCBI). The tRNAscan-SE 1.21 (Schattner et al. Citation2005) was also used to identify the tRNA gene regions. Reference sequences of related Annelida species were downloaded from GenBank, and the phylogenetic analysis was conducted using MEGA 7 (Kumar et al. Citation2016).
In the present study, the complete mitogenome of P. hoplura was determined with 17,707 bp in length (GenBank accession number MZ584801). The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov]. Its nucleotide composition was 29.1% A, 23.4% C, 14.3% G, and 33.2% T, and it showed a biased A + T ratio (62.2%). It consisted of two rRNA genes, 23 tRNA genes, 12 protein-coding genes, and one control region, and its architecture was similar to that of P. brevipalpa and P. websteri (Ye et al. Citation2021). Moreover, a second methionine tRNA (tRNA-Met) was also found in P. hoplura mitogenome (Zhong et al. Citation2008). Ye et al. (Citation2021) detected intergenic noncoding regions (NCR) in P. hoplura mitogenome. Interestingly, the atp8 gene was not found in the mitogenome of P. hoplura, even though it was found in the mitogenomes of P. brevipalpa and P. websteri. Seixas et al. (Citation2017) also reported the missing of atp8 gene in the mitogenome of Spirobranchus giganteus (Annelida: Serpulidae) (). Therefore, the further study is need to examine the existence and its function of atp8 gene among mitogenomes of polychaete species. Moreover, the nad2 (3′ end) and cox1 (5′ end) genes were overlapped in 23 bases (14 bases in P. brevipalpa and P. websteri).
Figure 1. Molecular phylogenetic relationships among published mitogenomes of related Polychaeta species. In the neighbor-joining tree, genetic distances were calculated by Kimura-2 parameter method based on concatenated nucleotide sequences of protein coding genes (PCGs). Numbers above the branches represent bootstrap values (2000 replicates).
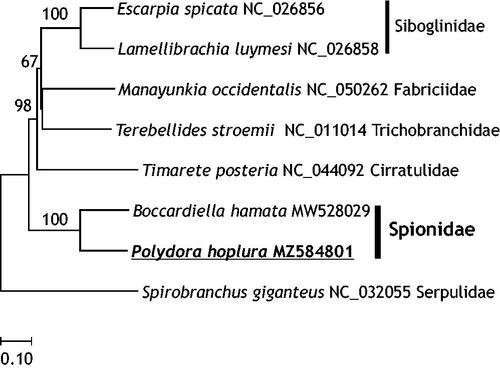
In summary, in the present study, we reported the complete mitogenome of P. hoplura and compared its interesting mitogenome features to those of other Polydora species. Our findings provide useful information for studying the evolution and phylogenetic relationships among polychaete species.
Author contributions
SJ Lee and S-R Lee carried out the experiment and the preparation of manuscript. SJ Lee presented the idea and conducted sampling, DNA experiments. S-R Lee conducted DNA experiment and analyzed the data.
Ethical approval
Experiments were performed in accordance with the recommendations of the Ethics Committee of the Research Project Management Regulations of National Fishery Products Quality Management Service (#20210501). These policies were enacted according to the National Research and Development Innovation Act.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The complete mitochondrial genome sequence of Polydora hoplura have been deposited in GenBank and openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/nuccore/MZ584801 under the accession no. MZ584801. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA785067, SRR17081562, and SAMN23526757 respectively.
Additional information
Funding
References
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
- Lee SJ, Kwon MG, Lee S-R. 2020. Molecular detection for two abalone shell-boring species Polydora haswelli and P. hoplura (Polychaeta, Spionidae) from Korea using 18S rDNA and cox1 markers. Ocean Sci J. 55(3):459–464.
- Radashevsky VI, Migotto AE. 2017. First report of the polychaete Polydora hoplura (Annelida: Spionidae) from North and South America and Asian Pacific. Mar Biodiv. 47(3):859–868.
- Sato-Okoshi W, Abe H, Nishitani G, Simon CA. 2017. And then there was one: Polydora uncinata and Polydora hoplura (Annelida: Spionidae), the problematic polydorid pest species represent a single species. J Mar Biol Ass. 97(8):1675–1684.
- Sato-Okoshi W, Okoshi K, Abe H, Li JY. 2013. Polydorid species (Polychaeta, Spionidae) associated with commercially important mollusk shells from eastern China. Aquaculture. 406–407:153–159.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.
- Seixas VC, de Moraes Russo CA, Paiva PC. 2017. Mitochondrial genome of the Christmas tree worm Spirobranchus giganteus (Annelida: Serpulidae) reveals a high substitution rate among annelids. Gene. 605:43–53.
- Won K-M, Kim B-H, Jin Y-G, Park Y-J, Son M-H, Cho M-Y, Park M-A, Park M-W. 2013. Infestation of the abalone, Haliotis discus hannai, by the Polydora under intensive culture conditions in Korea. Hangug Eobyeong Haghoeji. 26(3):139–148.
- Ye L, Yao T, Lu J, Jiang J, Bai C. 2021. Mitochondrial genomes of two Polydora (Spionidae) species provide further evidence that mitochondrial architecture in the Sedentaria (Annelida) is not conserved. Sci Rep. 11(1):13552.
- Zhong M, Struck TH, Halanych KM. 2008. Phylogenetic information from three mitochondrial genomes of Terebelliformia (Annelida) worms and duplication of the methionine tRNA. Gene. 416(1–2):11–21.
