Abstract
Mutations in mitochondrial DNA (mtDNA), especially in mitochondrial tRNA (mt-tRNAs) genes, play important roles in maternally inherited type 2 diabetes mellitus (T2DM), but the molecular mechanism remains unclear. In this study, two families with maternally transmitted T2DM are underwent clinical, genetic and molecular assessments. The mtDNA mutations are screened by direct sequencing. Furthermore, the phylogenetic conservation analysis and pathogenicity scoring system were used to evaluate the pathogenic status of mt-tRNA mutations. Interestingly, matrilineal relatives exhibit variable severity of DM, in particular, the age at onset of DM varies from 39 to 60 years, with an average of 50 years. Screening for the entire mitochondrial genomes identifies the existence of tRNAThr A15901G and C15926T mutations, as well as 59 variants belonging to mtDNA haplogroups D2 and C4c. Notably, the m.A15901G mutation is located at D-arm of tRNAThr, whereas the m.C15926T mutation resides in the anticodon loop of tRNAThr, both of these positions are well conserved and critical for tRNA functions. Thus, the m.A15901G and m.C15926T mutations may impair mitochondrial translation and lead to mitochondrial dysfunctions. However, the fail to identify any other functional variants indicate that mitochondrial haplogroup may not play a role in T2DM. Hence, tRNAThr A15901G and C15926T may be the novel mutations associated with T2DM.
Introduction
DM is a serious public health problem which remains a big challenging for physicians. Clinically, DM and its complications such as metabolic syndromes, cardiovascular diseases and foot problems contribute to its high mortality (Oguntibeju Citation2019; Lovic et al. Citation2020). In fact, T2DM is the most frequent type of DM worldwide (Magliano et al. Citation2019). Although great progress has been made toward T2DM, its pathogenicity is still elusive.
Recently, growing number of evidences indicate that T2DM is caused by genetic and environmental factors (Maassen et al. Citation2004, Citation2007). Some case-control studies implicate that T2DM could be maternally inherited (Avital et al. Citation2012; Fang et al. Citation2018), emphasizing the central roles of mitochondrial dysfunctions in this disease (Lin et al. Citation2021). Actually, mitochondria are found in most eukaryotic cells and involved in several biochemical processes, especially in generating ATP via oxidative phosphorylation (OXPHOS) (Wescott et al. Citation2019). Furthermore, as mtDNA lacks histones protection and is sensitive to oxidative stress, mtDNA has higher mutation rates than nuclear DNA (Madsen-Bouterse et al. Citation2010). There are several forms of T2DM-related mtDNA mutations: rearrangements such as large deletions (Ballinger et al. Citation1992) and point mutations in tRNAs or OXPHOS genes (Dabravolski et al. Citation2021). Among these, ND1 T3394C and T4216C, ND2 C5178A, ND3 A10398G, ND4L T10609C and C10676G, tRNALeu(UUR) A3243G and T3264C, tRNAIle T4291C, tRNAGlu T14709C and A14692G, tRNAGly T10003C have been identified as pathogenic mutations affecting T2DM predisposition (van den Ouweland et al. Citation1992; Suzuki et al. Citation1997; Damore et al. Citation1999; Wilson et al. Citation2004; Tang et al. Citation2006; Liu et al. Citation2015; Wang et al. Citation2016; Destiarani et al. Citation2020; Lalrohlui et al. Citation2020; Jiang et al. Citation2021). However, current knowledge of molecular mechanisms by which these mutations contribute to the pathogenesis of T2DM is widely unclear.
To explore the roles of mtDNA mutations for T2DM, this study described two families with T2DM, through PCR-Sanger sequencing, two tRNAThr mutations: m.A15901G and m.C15926T were identified. We noticed that all of these mutations occurred at evolutionary conserved positions, which may have functional impact on tRNAThr (Suzuki et al. Citation2011).
Materials and methods
Subjects and clinical assessments
Two families with DM (Family A and B) were ascertained in the Department of Occupational Therapy, Faculty of Health Science, Aino University (). Moreover, 330 healthy subjects (130 males and 200 females), aged from 38 to 58 years, with an average of 44 years old, were recruited as controls. The study protocols were approved by the Ethics Committee of Aino University. The written informed consent, as well as consent to publish these details had been obtained from all individuals.
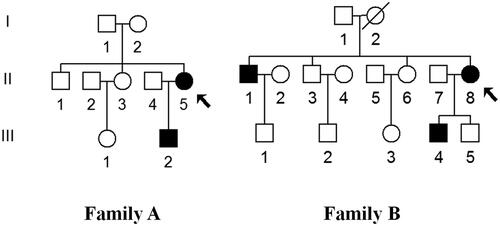
Figure 1. Two families with T2DM, affected individuals are indicated by filled symbols, arrows indicate the probands.
The diagnosis of T2DM is based on the criteria proposed by American Diabetes Association (Citation2010): Hemoglobin A1c (HbA1c) ≥6.5%; fasting plasma glucose (FPG) ≥126 mg/dl; an oral glucose tolerance test (OGTT) with a plasma glucose ≥200 mg/dl 2 hr after a 75-g glucose load.
Blood sample from each affected subject was collected in the morning between 7:00 AM and 10:00 AM after an overnight fast. The HbA1c level was measured by high-performance liquid chromatography (Bio-Rad, CA). While the serum FPG was determined by the regular laboratory methods (Beckman Coulter AU5800, Tokyo, Japan).
Mutational analysis of mitochondrial genomes
The genomic DNA of matrilineal relatives (Family A: II-5 and III-2; Family B: II-1, II-8 and III-4) were isolated from blood by using phenol-chloroform standard procedures (Lewin and Stewart-Haynes Citation1992). The individuals’ DNA fragments spanning the complete mtDNA genes were PCR amplified by 24 primers as described previously (Ding et al. Citation2020). After PCR amplification, the product was purified and sequenced by ABI PRISM 3100-Avant automated DNA sequencer using the BigDye Terminator Cycle reaction kit (version 1.1). The mtDNA mutations were screened by comparing the revised Cambridge reference sequence (rCRS, GenBank accession number: NC_012920.1) (Andrews et al. Citation1999).
Population screening
The m.A15901G and m.C15926T mutations were screened in 330 unrelated healthy subjects. The primers for amplification of tRNAThr gene were: forward: 5′-TGAAACTTCGGCTCACTCCT-3′, reverse: 5′- GAGTGGTTAATAGGGTGATAG-3′. After PCR and electrophoresis, fragment was purified and sequenced. The data was compared with the rCRS (GenBank accession number: NC_012920.1) to detect the existence of m.A15901G or m.C15926T mutation (Andrews et al. Citation1999).
Data analysis
To evaluate the pathogenicity of a specific mtDNA mutation, phylogenetic and haplogroup analyses were performed. Briefly, 17 species were used for conservation analysis as previously described (Ding et al. Citation2019). The Clustal software was applied for determining the conservation index (CI) of each mtDNA variant (Larkin et al. Citation2007), the CI value ≥75% was believed to have functional importance (Levin et al. Citation2013). Furthermore, mtDNA haplogroups were assigned by using PhyloTree (van Oven and Kayser Citation2009). We also searched for the presence of mtDNA mutations on Google Scholar and other databases including MITOMAP, mtDB or mtSNP to see whether the mutation was novel or not (Tanaka et al. Citation2004; Ingman and Gyllensten Citation2006; Bandelt et al. Citation2009; Lott et al. Citation2013).
Analysis of mt-tRNA secondary structure
MitotRNAdb, a database for analyzing mt-tRNA genes was employed to determine the stem and loop structure (Jühling et al. Citation2009). The interactions of tertiary structure for tRNA were based on the report by Suzuki et al. (Citation2011).
Assigning the pathogenicity
Due to the high mutation rate, distinguishing pathogenic mtDNA mutations from polymorphisms remained challenging (Yarham et al. Citation2010). The adjusted scoring system was used to determine the pathogenicity of m.A15901G and m.C15926T mutations (Yarham et al. Citation2011). Notably, if the total scores of a mutation ≤6, it was classified as ‘neutral polymorphism,’ if the scores were 7 ∼ 10, it belonged to ‘possibly pathogenic,’ if the scores ≥11, it was ‘definitely pathogenic.’
Results
Clinical presentations
Both the families had relatively high penetrances of T2DM (33.3% for Family A and 42.9% for Family B). In Family A, the proband (II-5) was a 65-year-old woman who went to Department of Occupational Therapy, Faculty of Health Science, Aino University for treatment of T2DM. As shown in , the HbA1c level, as well as the OGTT results strongly indicated that she was a diabetic carrier, further genetic counseling suggested that she developed T2DM 10 years ago, and the family history indicated a maternal inheritance. Moreover, we noticed that the family member (III-3) and the proband (II-5) had glucose intolerance.
Table 1. Summary of clinical and biochemical data for several members in two families with DM.
In Family B, the proband (II-8) was a 66-years-old woman who went to the Department of Occupational Therapy, Faculty of Health Science, Aino University for regular treatment of DM. She suffered from DM when she was 58. Among 7 matrilineal relatives, 3 subjects developed DM. The age at onset of DM ranged from 41 to 60 years (mean age: 53). Moreover, these members showed no other clinical abnormalities, including cancer, hearing loss, neurological or infection disorders.
Screening for mitochondrial mutations
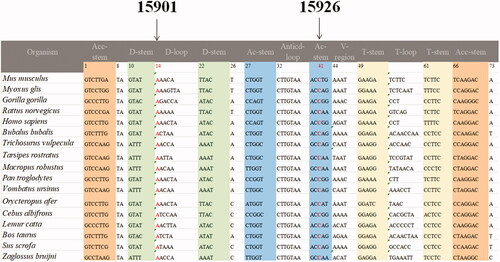
To explore the molecular basis of T2DM, we screened the mtDNA mutations of matrilineal relatives (Family A: II-5 and III-2; Family B: II-1, II-8 and III-4) by using PCR and sequencing. As shown in , compared with the rCRS, which allowed to be assigned to haplogroup D2 for Family A and haplogroup C4c for Family B (van Oven and Kayser Citation2009). Among these, there were 16 known variants in D-loop, four variants in 12S rRNA, four variants in 16S rRNA, two mutations in tRNAThr (m.A15901G and m.C15926T), while others were localized in OXPHOS-genes (Owen and McCarthy Citation2007). Moreover, ten missense mutations were identified: ND1 C3497T (Ala to Val) and T4216C (Tyr to His), ND2 G4491A (Val to Ile), A8 C8414T (Leu to Phe), A6 A8701G (Thr to Ala) and A8860G (Thr to Ala), ND3 A10398G (Thr to Ala), ND5 G13928C (Ser to Thr), CytB C14766T (Thr to Ile) and A15326G (Thr to Ala). These variants in RNAs and polypeptides were further evaluated by phylogenetic analysis and sequences from other 16 vertebrates, including mouse (Bibb et al. Citation1981), bovine (Gadaleta et al. Citation1989) and Xenopus laevis (Roe et al. Citation1985). We found that except for the m.A15901G and m.C15926T mutations (), other variants were not well conserved. We also screened the presence of m.A15901G or m.C15926T mutation in 330 controls, as shown in , after electrophoresis, the size of PCR product spanning the entire tRNAThr gene was 1,126-bp. However, none of the healthy subjects harbored these mutations.
Figure 2. Identification of m.A15901G and m.C15926T mutations by direct sequencing analysis. MT: mutant; WT: wild type.
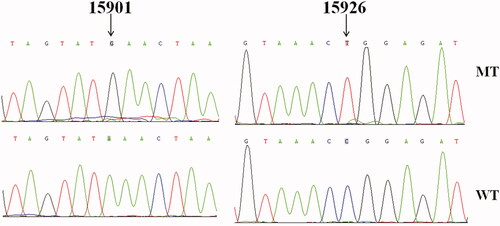
Figure 3. PCR amplification of mt-tRNAThr gene in control subjects, arrow indicates the PCR product, which is 1,126-bp.
Table 2. mtDNA mutations in two families with T2DM.
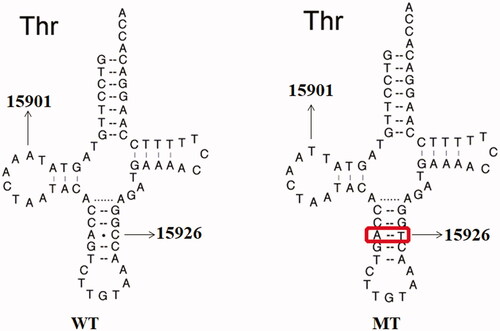
As shown in and , the homoplasmic m.A15901G mutation occurred at position 14 in the D-arm of tRNAThr, which was extremely conserved from different species. Moreover, the m.C15926T mutation was located at position 41 in the anticodon stem of tRNAThr. It was interesting to note that the m.C15926T mutation created a novel base-pairing (29 A-41T) and may result a failure in tRNA metabolism ().
M.A15901G and m.C15926T mutations were ‘possibly pathogenic’ for T2DM
According to the revised pathogenicity scoring system (Yarham et al. Citation2011), the total scores of m.A15901G and m.C15926T mutations were both 10 points and belonged to ‘possibly pathogenic’ for T2DM ().
Table 3. The predicted pathogenicity of tRNAThr A15901G and C15926T mutations.
Discussion
In the present study, two families with maternally inherited T2DM were underwent clinical and molecular evaluations. T2DM was only presented in matrilineal lineage of these pedigrees, suggesting that mtDNA mutations were the molecular basis for this disorder. The age at onset of DM varied from 39 to 60 years, with an average of 50 years. The variable age at onset of DM in these pedigrees suggested that nuclear genes, environmental factors and epigenetic modifications may contribute to T2DM progression, as in the case of m.T3394C mutation (You et al. Citation2021).
Sequence analysis of the mitochondrial genomes in Family A and B revealed the presence of two tRNAThr mutations, together with sets of polymorphisms belonging to mitochondrial haplogroup D2 and C4c, respectively (van Oven and Kayser Citation2009). In fact, the m.A15901G mutation resided at position 14 in the D-arm of tRNAThr, which was conserved from various species. Furthermore, m.A15901G mutation occurred at 14 A-8T interaction, which was served as one of determinants for tRNA recognition by cognate aminoacyl-tRNA synthetases (Figuccia et al. Citation2021). Hence, this mutation may affect aminoacylation ability, as well as the steady-state level of tRNAThr (Zheng et al. Citation2020). Notably, the m.A5814G which occurred at the same position of tRNACys had been regarded as a pathogenic mutation for mitochondrial encephalopathy (Santorelli et al. Citation1997; Sternberg et al. Citation1998). Importantly, biochemical analysis of the muscle derived from the patients carrying the m.A5814G mutation revealed significant reductions in the activities of complexes I and IV (Scuderi et al. Citation2007). Thus, it can be speculated that the m.A15901G, which was similar to the m.A5814G mutation, may also impair respiratory chain functions and lead to mitochondrial dysfunctions.
Furthermore, the homoplasmic m.C15926T mutation created a new base-pairing (29 A-41T) in the anticodon stem of tRNAThr. Interestingly, the 3272delT which occurred at the same position in tRNALeu(UUR) had been found to be associated with mitochondrial encephalomyopathy (Shoffner et al. Citation1995). In fact, nucleotide at position 41 of tRNAs was often modified, thereby contributing to the structural formation and stabilization of tRNAs (Ding et al. Citation2021). Therefore, the m.C15926T mutation may alter the tRNAThr post-transcriptionally modification and lead to the failure in tRNA metabolism (Wong et al. Citation2020).
Heteroplasmic mtDNA mutation had been traditionally considered as an important factor for pathogenicity. However, there were some pathogenic mtDNA mutations that were in homoplasmic forms (McFarland et al. Citation2004; Jia et al. Citation2019; Zhao et al. Citation2019). These mutations were considered relatively mild and may require additional factors to produce a clinical phenotype (Cai et al. Citation2021; Cataldi et al. Citation2021; Dixon et al. Citation2021).
We believed that m.A15901G and m.C15926T were pathogenic mutations on the following lines of evidence: (1) these mutations presented only in DM patients but were absent in controls; (2) the A-to-G and C-to-T at positions 15901 and 15926 were extremely conserved from different species and predicted to affect the secondary structure of tRNAThr; (3) these mutations segregated in the maternal lineage and healthy subjects.
Based on these observations, the molecular mechanisms underlying m.A15901G and m.C15926T mutations may be as follows: these mutations influenced the structure of tRNAThr and caused the failure in tRNA metabolism, which may subsequently affect mitochondrial translation and respiratory chain functions (Wallace Citation2018). These events would lead to mitochondrial dysfunctions including the drop in ATP and mitochondrial membrane potential, increasing ROS generation, which will cause β-cell apoptosis and contributed to T2DM progression (Pinti et al. Citation2019).
In conclusion, this report suggested that m.A15901G and m.C15926T mutations were associated with DM. The limitation of current study was the relatively small sample size, further studies including more patients were needed to verify this conclusion.
Authors contribution
MA and MC designed the study, UT and KT performed the clinical and molecular assessment, MK analyzed the data, MA and MC wrote the draft and revised it critically for intellectual content, MC approved the final version of this manuscript, all authors agreed to be accountable for all aspects of the work.
Acknowledgement
We thanked the patients and control subjects for participating for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession NC_012920.1.
References
- American Diabetes Association. 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care. 33 (Suppl. 1):S62–S69.
- Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. 1999. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 23(2):147.
- Avital G, Buchshtav M, Zhidkov I, Tuval Feder J, Dadon S, Rubin E, Glass D, Spector TD, Mishmar D. 2012. Mitochondrial DNA heteroplasmy in diabetes and normal adults: role of acquired and inherited mutational patterns in twins. Hum Mol Genet. 21(19):4214–4224.
- Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC. 1992. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1(1):11–15.
- Bandelt HJ, Salas A, Taylor RW, Yao YG. 2009. Exaggerated status of “novel” and “pathogenic” mtDNA sequence variants due to inadequate database searches. Hum Mutat. 30(2):191–196.
- Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA. 1981. Sequence and gene organization of mouse mitochondrial DNA. Cell. 26(2):167–180.
- Cai Y, Yao H, Sun Z, Wang Y, Zhao Y, Wang Z, Li L. 2021. Role of NFAT in the progression of diabetic atherosclerosis. Front Cardiovasc Med. 8:635172.
- Cataldi S, Costa V, Ciccodicola A, Aprile M. 2021. PPARγ and diabetes: beyond the genome and towards personalized medicine. Curr Diab Rep. 21(6):18.
- Dabravolski SA, Orekhova VA, Baig MS, Bezsonov EE, Starodubova AV, Popkova TV, Orekhov AN. 2021. The role of mitochondrial mutations and chronic inflammation in diabetes. Int J Mol Sci. 22(13):6733.
- Damore ME, Speiser PW, Slonim AE, New MI, Shanske S, Xia W, Santorelli FM, DiMauro S. 1999. Early onset of diabetes mellitus associated with the mitochondrial DNA T14709C point mutation: patient report and literature review. J Pediatr Endocrinol Metab. 12(2):207–213.
- Destiarani W, Mulyani R, Yusuf M, Maksum IP. 2020. Molecular dynamics simulation of T10609C and C10676G mutations of mitochondrial ND4L gene associated with proton translocation in type 2 diabetes mellitus and cataract patients. Bioinform Biol Insights. 14:1177932220978672.
- Ding Y, Teng YS, Zhuo GC, Xia BH, Leng JH. 2019. The mitochondrial tRNAHis G12192A mutation may modulate the clinical expression of deafness-associated tRNAThr G15927A mutation in a Chinese pedigree. Curr Mol Med. 19(2):136–146.
- Ding Y, Ye YF, Li MY, Xia BH, Leng JH. 2020. Mitochondrial tRNAAla 5601C > T variant may affect the clinical expression of the LHON related ND4 11778G > A mutation in a family. Mol Med Rep. 21(1):201–208.
- Ding Y, Zhuo G, Guo Q, Li M. 2021. Leber’s hereditary optic neuropathy: the roles of mitochondrial transfer RNA variants. PeerJ. 9:e10651.
- Dixon ED, Nardo AD, Claudel T, Trauner M. 2021. The Role of Lipid Sensing Nuclear receptors (PPARs and LXR) and metabolic lipases in obesity, diabetes and NAFLD. Genes. 12(5):645.
- Fang H, Hu N, Zhao Q, Wang B, Zhou H, Fu Q, Shen L, Chen X, Shen F, Lyu J. 2018. mtDNA haplogroup N9a increases the risk of type 2 diabetes by Altering mitochondrial function and intracellular mitochondrial signals. Diabetes. 67(7):1441–1453.
- Figuccia S, Degiorgi A, Ceccatelli Berti C, Baruffini E, Dallabona C, Goffrini P. 2021. Mitochondrial aminoacyl-tRNA synthetase and disease: the yeast contribution for functional analysis of novel variants. Int J Mol Sci. 22(9):4524.
- Gadaleta G, Pepe G, De Candia G, Quagliariello C, Sbisà E, Saccone C. 1989. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 28(6):497–516.
- Ingman M, Gyllensten U. 2006. mtDB: human mitochondrial genome database, a resource for population genetics and medical sciences. Nucleic Acids Res. 34(Database issue):D749–51.
- Jia Z, Zhang Y, Li Q, Ye Z, Liu Y, Fu C, Cang X, Wang M, Guan MX. 2019. A coronary artery disease-associated tRNAThr mutation altered mitochondrial function, apoptosis and angiogenesis. Nucleic Acids Res. 47(4):2056–2074.
- Jiang Z, Teng L, Zhang S, Ding Y. 2021. Mitochondrial ND1 T4216C and ND2 C5178A mutations are associated with maternally transmitted diabetes mellitus. Mitochondrial DNA A DNA Mapp Seq Anal. 32(2):59–65.
- Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. 2009. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 37(Database issue):D159–62.
- Lalrohlui F, Zohmingthanga J, Hruaii V, Kumar NS. 2020. Genomic profiling of mitochondrial DNA reveals novel complex gene mutations in familial type 2 diabetes mellitus individuals from Mizo ethnic population, Northeast India. Mitochondrion. 51:7–14.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23(21):2947–2948.
- Levin L, Zhidkov I, Gurman Y, Hawlena H, Mishmar D. 2013. Functional recurrent mutations in the human mitochondrial phylogeny: dual roles in evolution and disease. Genome Biol Evol. 5(5):876–890.
- Lewin HA, Stewart-Haynes JA. 1992. A simple method for DNA extraction from leukocytes for use in PCR. Biotechniques. 13(4):522–524.
- Lin L, Zhang D, Jin Q, Teng Y, Yao X, Zhao T, Xu X, Jin Y. 2021. Mutational analysis of mitochondrial tRNA genes in 200 patients with type 2 diabetes mellitus. Int J Gen Med. 14:5719–5735.
- Liu H, Li R, Li W, Wang M, Ji J, Zheng J, Mao Z, Mo JQ, Jiang P, Lu J, et al. 2015. Maternally inherited diabetes is associated with a homoplasmic T10003C mutation in the mitochondrial tRNA(Gly) gene. Mitochondrion. 21:49–57.
- Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V, Wallace DC. 2013. mtDNA variation and analysis using mitomap and mitomaster. Curr Protoc Bioinformatics. 44(123):1.23–1-26.
- Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. 2020. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 18(2):104–109.
- Maassen JA, Romijn JA, Heine RJ. 2007. Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia. 50(10):2036–2041.
- Maassen JA, 'T Hart LM, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, Raap AK, Janssen GM, Lemkes HH. 2004. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 53(Suppl 1):S103–S9.
- Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. 2010. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 13(6):797–805.
- Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, Tabesh M, Koye DN, Shaw JE. 2019. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 366:l5003.
- McFarland R, Schaefer AM, Gardner JL, Lynn S, Hayes CM, Barron MJ, Walker M, Chinnery PF, Taylor RW, Turnbull DM. 2004. Familial myopathy: new insights into the T14709C mitochondrial tRNA mutation. Ann Neurol. 55(4):478–484.
- Oguntibeju OO. 2019. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 11(3):45–63.
- Owen KR, McCarthy MI. 2007. Genetics of type 2 diabetes. Curr Opin Genet Dev. 17(3):239–244.
- Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A, Hollander JM. 2019. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 316(2):E268–E285.
- Roe BA, Ma DP, Wilson RK, Wong JF. 1985. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 260(17):9759–9774.
- Santorelli FM, Siciliano G, Casali C, Basirico MG, Carrozzo R, Calvosa F, Sartucci F, Bonfiglio L, Murri L, DiMauro S. 1997. Mitochondrial tRNA(Cys) gene mutation (A5814G): a second family with mitochondrial encephalopathy. Neuromuscul Disord. 7(3):156–159.
- Scuderi C, Borgione E, Musumeci S, Elia M, Castello F, Fichera M, Davidzon G, DiMauro S. 2007. Severe encephalomyopathy in a patient with homoplasmic A5814G point mutation in mitochondrial tRNACys gene. Neuromuscul Disord. 17(3):258–261.
- Shoffner JM, Bialer MG, Pavlakis SG, Lott M, Kaufman A, Dixon J, Teichberg S, Wallace DC. 1995. Mitochondrial encephalomyopathy associated with a single nucleotide pair deletion in the mitochondrial tRNALeu(UUR) gene. Neurology. 45(2):286–292.
- Sternberg D, Danan C, Lombès A, Laforêt P, Girodon E, Goossens M, Amselem S. 1998. Exhaustive scanning approach to screen all the mitochondrial tRNA genes for mutations and its application to the investigation of 35 independent patients with mitochondrial disorders. Hum Mol Genet. 7(1):33–42.
- Suzuki T, Nagao A, Suzuki T. 2011. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 45:299–329.
- Suzuki Y, Suzuki S, Hinokio Y, Chiba M, Atsumi Y, Hosokawa K, Shimada A, Asahina T, Matsuoka K. 1997. Diabetes associated with a novel 3264 mitochondrial tRNA(Leu)(UUR) mutation. Diabetes Care. 20(7):1138–1140.
- Tanaka M, Takeyasu T, Fuku N, Li-Jun G, Kurata M. 2004. Mitochondrial genome single nucleotide polymorphisms and their phenotypes in the Japanese. Ann NY Acad Sci. 1011:7–20.
- Tang DL, Zhou X, Li X, Zhao L, Liu F. 2006. Variation of mitochondrial gene and the association with type 2 diabetes mellitus in a Chinese population. Diabetes Res Clin Pract. 73(1):77–82.
- van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. 1992. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1(5):368–371.
- van Oven M, Kayser M. 2009. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 30(2):E386–94.
- Wallace DC. 2018. Mitochondrial genetic medicine. Nat Genet. 50(12):1642–1649.
- Wang M, Liu H, Zheng J, Chen B, Zhou M, Fan W, Wang H, Liang X, Zhou X, Eriani G, et al. 2016. A deafness- and diabetes-associated tRNA mutation causes deficient pseudouridinylation at position 55 in tRNAGlu and mitochondrial dysfunction. J Biol Chem. 291(40):21029–21041.
- Wescott AP, Kao JPY, Lederer WJ, Boyman L. 2019. Voltage-energized calcium-sensitive ATP production by mitochondria. Nat Metab. 1(10):975–984.
- Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, et al. 2004. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 306(5699):1190–1194.
- Wong LC, Chen T, Wang J, Tang S, Schmitt ES, Landsverk M, Li F, Wang Y, Zhang S, Zhang VW, et al. 2020. Interpretation of mitochondrial tRNA variants. Genet Med. 22(5):917–926.
- Yarham JW, Al-Dosary M, Blakely EL, Alston CL, Taylor RW, Elson JL, McFarland R. 2011. A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum Mutat. 32(11):1319–1325.
- Yarham JW, Elson JL, Blakely EL, McFarland R, Taylor RW. 2010. Mitochondrial tRNA mutations and disease. Wiley Interdiscip Rev Rna. 1(2):304–324.
- You X, Huang X, Bi L, Li R, Zheng L, Xin C. 2021. Clinical and molecular features of two diabetes families carrying mitochondrial ND1 T3394C mutation. Ir J Med Sci. 2021:4. Online ahead of print.
- Zhao X, Cui L, Xiao Y, Mao Q, Aishanjiang M, Kong W, Liu Y, Chen H, Hong F, Jia Z, et al. 2019. Hypertension-associated mitochondrial DNA 4401A > G mutation caused the aberrant processing of tRNAMet, all 8 tRNAs and ND6 mRNA in the light-strand transcript. Nucleic Acids Res. 47(19):10340–10356.
- Zheng J, Bai X, Xiao Y, Ji Y, Meng F, Aishanjiang M, Gao Y, Wang H, Fu Y, Guan MX. 2020. Mitochondrial tRNA mutations in 887 Chinese subjects with hearing loss. Mitochondrion. 52:163–172.