Abstract
The chloroplast genome of Zingiber striolatum Diels was sequenced using the MGI paired-end sequencing method and assembled. The chloroplast genome was 163,711 bp in length, containing a large single-copy (LSC) region of 88,205 bp, a small single-copy (SSC) region of 15,750 bp, and two inverted repeat (IR) regions of 29,752 bp. The overall GC content was 36.1%, whereas the corresponding value in the IR regions was 41.1%, which was higher than that in the LSC region (33.8%) and SSC region (29.6%). A total of 136 complete genes were annotated in the chloroplast genome of Z. striolatum, including 87 protein-coding genes (79 protein-coding gene species), 40 tRNA genes (29 tRNA species), and 8 rRNA genes (4 rRNA species). A phylogenetic tree was constructed using the maximum likelihood (ML) method, and the results showed that the phylogeny of Zingiber was well resolved with high support values, and Z. striolatum was sister to Z. mioga. The assembly and sequence analysis of the chloroplast genome can provide a basis for developing high-resolution genetic makers.
Introduction
Zingiber striolatum Diels (Diels 1901) is a perennial herb belonging to the genus Zingiber from the family Zingiberaceae (Tong Citation1998). Z. striolatum is a local vegetable, commonly known as Yang-He in China. The edible part of Z. striolatum is flowers (Huang et al. Citation2019). It is widely distributed, predominantly in many Chinese provinces with an altitude of about 800 m (An editorial committee of flora of China 1981; Huang et al. Citation2019). Although Z. striolatum could be cultivated with artificial plots in residential areas (e.g. in gardens), it is originally harvested from the wild. The plant height of Z. striolatum is 1–1.5 m (Deng et al. Citation2018). Z. striolatum has a cylindrical rhizome and an aromatic smell like other ginger species. Z. striolatum has high medicinal and edible value. The characteristics of Zingiber vegetative organs are highly similar, so it is extremely difficult to distinguish Z. striolatum from other Zingiber species (Theerakulpisut et al. Citation2012). DNA barcoding has been proved to be an automated, rapid, and accurate biological tool for species identification (Chen et al. Citation2010; Hebert et al. Citation2003). The informative sites of DNA barcoding markers are vital for the efficiency of species identification. Divergent hotspots for species identification have been reported in the subfamily Zingiberoideae (Li et al. Citation2021). However, the chloroplast genome of Z. striolatum has not yet been reported. In this study, we sequenced and assembled the complete chloroplast genome of Z. striolatum, which will contribute to understanding the population genetics of Z. striolatum and the chloroplast evolutionary dynamics of the genus Zingiber.
Plant materials
The plant materials of Z. striolatum sequenced in this study were collected from Xishuangbanna Botanical Garden, Jinghong City, Yunnan Province (21°41′N, 101°25′E) (). This species is common in Southwest Yunnan, and no permission is required to collect these samples. Fresh and healthy leaves were dried with silica gel and preserved for DNA extraction. A specimen was deposited at the Herbarium of Chongqing University of Arts and Sciences (www.cqwu.net, Huamin Liu, [email protected]) under the voucher number Li_Z033.
Methods
The modified sucrose gradient centrifugation method was used to extract total genomic DNA from silica-gel-dried leaves (Li et al. Citation2012). The concentration and quantity of isolated genomic DNA were assessed using NanoDrop 2000 micro spectrometer (Wilmington, DE, USA). The isolated genomic DNA was stored in the −80 °C refrigerator of the Key Laboratory of the College of Landscape Architecture and Life Science. A paired-end DNA library with an insert size of 350 bp was prepared using the MGI Easy PCR-Free DNA Library Prep kit (MGI-TECH), and then was sequenced by the MGI DNBSEQ-T7 platform (MGI-TECH, ShenZhen, China). A total of 5.0 Gbp raw data were produced. To obtain clean data, the adaptor and low-quality sequences were removed. The software NOVOPlasty v3.5 was used to assemble the whole chloroplast genome. The software Genius v11.0.4 was used to annotate the chloroplast genome, the result of which was corrected using Z. Officinal (MW602894) as a reference genome.
The complete chloroplast genome sequences of Z. striolatum and 8 other Zingiber species were used to construct a phylogenetic tree, in which Kaempferia galangal was used as the outgroup. All the nine chloroplast genome sequences were aligned by MUSCLE with the default settings and refined manually. Then the ML tree was constructed using IQ-TREE with the default settings, including the GTRGAMMA model along with 1000 bootstrap (BS) replicates (Nguyen et al. Citation2015). All the above analyses were carried out using Geneious v11.0.4.
Results
The complete chloroplast genome of Z. striolatum (National Genomics Data Center accession number ON646165) was characterized from MGI paired-end sequencing. The chloroplast genome of Z. striolatum was 163,711 bp in length, containing one small single-copy (SSC) region of 15,750 bp, one large single-copy (LSC) region of 88,205 bp, and two inverted repeats (IRA and IRB) of 29,752 bp (). The overall GC content was 36.1%, whereas the corresponding value in the IR regions was 41.1%, which was higher than that in the LSC region (33.8%) and the SSC region (29.6%). The sequenced chloroplast genome contained 136 predicted functional genes, including 87 protein-coding genes (79 protein-coding gene species), 40 tRNA genes (29 tRNA species), and 8 rRNA genes (4 rRNA species).
Figure 2. The structure of Zingiber striolatum chloroplast genome. The genes inside and outside of the circle are transcribed in the clockwise and counterclockwise directions, respectively. Genes belonging to different functional groups are shown in different colors. The darker gray area in the inner circle indicates the GC content and the lighter gray indicates the AT content of the chloroplast genome. The thick lines indicate the extent of the inverted repeats (IRA and IRB) that separate the chloroplast genome into the small single-copy (SSC) and large single-copy (LSC) regions.
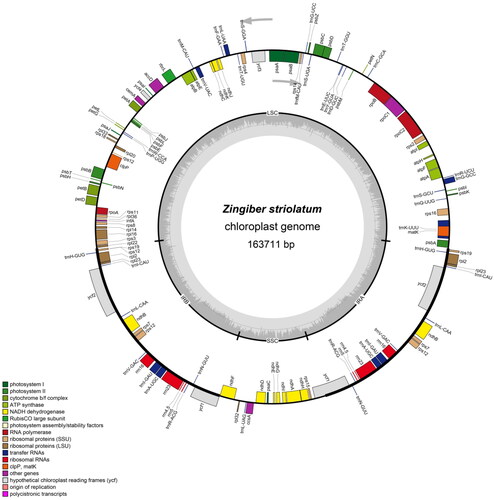
Figure 3. Phylogenetic tree of Zingiber inferred using maximum likelihood (ML) based on the complete chloroplast genome sequences. GenBank accession numbers: Z. striolatum (ON646165), Z. recurvatum (MT473712), Z. mioga (NC_057615), Z. officinale (MW602894), Z. montanum (MW801386), Z. corallinum (MW801385), Z. spectabile (NC_020363), Z. zerumbet (MK262726), and Kaempferia gaalanga (MK209001).
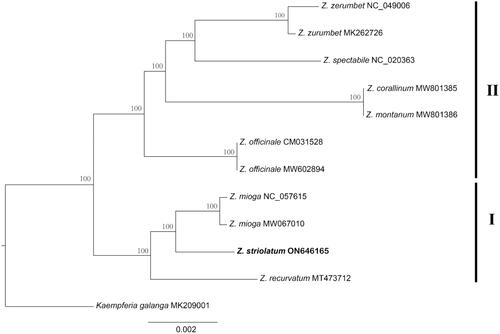
Based on the chloroplast genome dataset, we generated a well-resolved phylogenetic tree of Zingiber (Figure3). The support values of all the branches were robust (BS = 100%). Two well-supported lineages were recovered. Clade I contained Z. recurvatum, which was sister to Z. mioga and Z. striolatum. Clade II was composed of Z. officinale + ((Z. corallinum + Z. montanum) + (Z. zerumbet + Z. spectabile)).
Discussion and conclusion
The genome size (163,711 bp), overall GC content (36.1%), genome quadripartite structure, and gene composition (including the protein-coding, tRNA, and rRNA genes) in the Z. striolatum chloroplast genome were highly similar to those in other Zingiber chloroplast genomes (Li et al. Citation2021). These results suggested that the chloroplast genomes of Zingiber species were highly conserved at the genus level. Overall, the phylogeny of Zingiber is congruent with the previous studies (Theerakulpisut et al. Citation2012; Li et al. Citation2021). Our phylogeny showed that Z. recurvatum was closely related to Z. mioga, which is consistent with the published molecular phylogeny based on chloroplast genome data (Li et al. Citation2021). Our results showed that Z. zerumbet was sister to Z. spectabile. Previous trees constructing using using either chloroplast genome sequences or nuclear ribosomal internal transcribed spacer (ITS) regions showed congruence of species relationship of Z. zerumbet and Z. spectabile (Theerakulpisut et al. Citation2012; Li et al. Citation2021). However, the support values of each branch within the phylogeny of Zingiber based on chloroplast genome data were higher than that based on nuclear genome data. These results indicated that the chloroplast genome is more effective in resolving the phylogenetic relationships among Zingiber species.
The assembly and sequence analysis of the chloroplast genome can provide a basis for developing high-resolution genetic makers, and also lay a foundation for subsequent studies on plant population genetics and genetic diversity.
Author contributions
Hong-Lei Li and Linzheng Liao conceived the study. Shuming Tian and Dongzhu Jiang performed the experiments. Linzheng Liao, Xiao Wang, Shuming Tian, Yuepeng Wan, Qinhong Liao and Qiang Li contributed reagents/materials/analysis tools and analyzed the data. Hong-Lei Li, Shuming Tian and Linzheng Liao wrote the paper. Hong-Lei Li, Shuming Tian and Linzheng Liao edited the paper.
Acknowledgement
The authors would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/nuccore/ON646165) under the accession no. ON646165. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA842832, SRR19426750, and SAMN28688325 respectively.
Additional information
Funding
References
- Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, et al. 2010. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One. 5(1):e8613.
- Deng K, Deng R, Fan J, Chen E. 2018. Transcriptome analysis and development of simple sequence repeat (SSR) markers in Zingiber striolatum Diels. Physiol Mol Biol Plants. 24(1):125–134.
- Hebert P, Cywinska A, Ball SL, Dewaard JR. 2003. Biological identifications through DNA barcodes. Proc R Soc Lond B. 270(1512):313–321.
- Huang Z, Xie L, Wang H, Zhong J, Li Y, Liu J, Ou Z, Liang X, Li Y, Huang H, et al. 2019. Geographic distribution and impacts of climate change on the suitable habitats of Zingiber species in China. Ind Crops Prod. 138:111429.
- Li HL, Wu L, Dong Z, Jiang Y, Jiang S, Xing H, Wu Z. 2021. Haplotype-resolved genome of diploid ginger (Zingiber officinale) and its unique gingerol biosynthetic pathway. Hortic Res. 8(1):13.
- Li XW, Hu ZG, Lin XH, Li Q, Gao HH, Luo GA, Chen SL. 2012. High-throughput pyrosequencing of the complete chloroplast genome of Magnolia officinalis and its application in species identification. Acta Pharm Sin. 47(1):124–130.
- Li DM, Li J, Wang DR, Xu YC, Zhu GF. 2021. Molecular evolution of chloroplast genomes in subfamily Zingiberoideae (Zingiberaceae). BMC Plant Biol. 21(1):558.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum- likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Theerakulpisut P, Triboun P, Mahakham W, Maensiri D, Khampila J, Chantaranothai P. 2012. Phylogeny of the genus Zingiber (Zingiberaceae) based on nuclear ITS sequence data. Kew Bull. 67(3):389–395.
- Tong SQ. 1998. Revision and additional notes of Zingiberaceae of Yunnan. China. Bull Bot Res. 25:460–471.
- Huang ZB, Xie LN, Xu YY, Zhao K, Li XT, Zhong JB, Lu YJ, Xu XT, Goodin SS, Zhang K, et al. 2021. Essential oils from Zingiber striolatum diels attenuate inflammatory response and oxidative stress through regulation of MAPK and NF-κB signaling pathways. Antioxidants. 10(12):2019.

