Abstract
Striga asiatica (L.) Kuntze 1891 is a hemiparasitic plant native to Asia and Africa. It is invasive and causes yield losses in crops such as corn, rice and sorghum. Lack of chloroplast genomic data has limited research into its obligate parasitic lifestyle. In this study, the complete chloroplast genome of Striga asiatica was sequenced and characterized. It is a quadripartite structure with a total length of 191,085 bp and a GC content of 37.86%. It has a large single copy region (LSC) of 51,406 bp, a small single copy region (SSC) of 273 bp, and two copies of the reverse repeat sequence (IRA and IRB) of 69,703 bp. A total of 122 protein-coding genes, 8 rRNA genes, and 44 tRNA genes were annotated in the chloroplast genome. There were a lot of ndh gene deletions and pseudogenizations in this chloroplast genome. For example, ndhA, D, E, H, I, and K were all pseudogenes because they were missing the 5′ end start codon. ndhB, C, and J had shorter gene lengths than their homologs, and ndhF and ndhG were missing genes. The phylogenetic tree reveals that all Striga species form a clade, and a bootstrap value of 100 indicates that S. asiatica is closely related to Striga hermonthica and Striga sepera. The comprehensive chloroplast genomic resource of S. asiatica would assist researchers in comprehending hemiparasitic mechanisms, molecular markers, and evolutionary patterns of the genus Striga.
Introduction
Striga asiatica (L.) Kuntze 1891, a member of the Orobanchaceae family, is a hemiparasitic plant native to Asia and Sub-Saharan Africa (Cochrane and Press Citation1997). It is recognized as an invasive plant in many countries because its parasitism damages several major crops, including corn, rice, and sorghum, resulting in considerable yield losses (Spallek et al. Citation2013). In China and India, S. asiatica is used as folk medicine to treat high blood pressure, poor appetite, jaundice, and fever (Kakpure and Rothe Citation2012). Although the nuclear genome of S. asiatica has been sequenced (Yoshida et al. Citation2019), the lack of chloroplast genomic material has hampered research into Striga asiatica, particularly in terms of its obligate parasitic lifestyle. In this study, we obtained the complete chloroplast genome of S. asiatica using next-generation sequencing technology, characterized its basic features, and analyzed its phylogenetic relationship with other plants in the family Orobanchaceae.
Materials and methods
The fresh leaves of S. asiatica () were collected from the Yulin Normal University Horticultural Germplasm Center located in Yulin, Guangxi, China (110.185°E, 22.669°N). The voucher specimen (DJJ-YNU-001) was deposited in the Herbarium of Yulin Normal University (https://syy.ylu.cn/index.html, Yulin Zhu, [email protected]). The total genomic DNA was isolated using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Biozeron (Biozeron, Shanghai, China) was entrusted with the library preparation and sequencing utilizing the Illumina Hiseq 4000 sequencing technology (Illumina, San Diego, CA). The adapter removal and quality control of raw data was conducted by Trimmomatic v0.39 by using the following parameters: ‘ILLUMINACLIP: TruSeq3-PE-2.fa:2:30:10:8:true LEADING:4 TRAILING:4 SLIDINGWINDOW:4:110 MINLEN:100.’ The de novo assembling of the complete chloroplast genome was achieved by using the GetOrganelle toolkit with default parameters (Jin et al. Citation2020). To assess the quality of the resulting chloroplast genome assembly, we applied the ‘evaluate_assembly_using_mapping.py’ script from the aforementioned toolkit, which allowed us to accurately calculate the overall coverage depth (Figure S1). With the help of CPGAVAS2 (Shi et al. Citation2019) and GeSeq (Tillich et al. Citation2017), the chloroplast genome of the hemiparasitic Pedicularis hallaisanensis (Orobanchaceae) was used as a guide to annotate the chloroplast genome of Striga asiatica in detail. The final sequence and annotation file was summitted to NCBI with the accession of ON652844.1. The OGDRAW (Greiner et al. Citation2019) was employed to convert the chloroplast genome annotation into graphical map (). To visualize the structures of splicing genes within the chloroplast genome of S. asiatica (Figure S2 and S3), we utilized the CPGView tool (Liu et al. Citation2023). The study utilized a total of 16 complete chloroplast genome sequences from the Orobanchaceae family and 2 from other families in order to reconstruct a phylogenetic tree and establish the taxonomic status of S. asiatica. MAFFT v7 (Rozewicki et al. Citation2019) was used to align the single-copy orthologue genes of all chloroplast genomes with default parameters, and the ModelFinder in IQ-TREE v1.6.10 (Nguyen et al. Citation2015) was used to perform model detection. With the best-fit model TVM + F + I + G4 and two outgroup species from the Solanaceae, Agastache rugosa and Callicarpa americana, the maximum likelihood (ML) tree with 1000 bootstraps was constructed ().
Figure 1. The morphological features of Striga asiatica. S. asiatica is an annual and semi-parasitic species, typified by its slender, striated leaves, spikes or individual blooms of yellow (occasionally tinged with red or white) with a deeply arched corolla tube, and an oval seed capsule that endures within a calyx bearing ten ridges. The photo was captured by RiZhen Bao in May of 2022 at Yulin Normal College, with no copyright concerns.

Figure 2. Chloroplast genome map of Striga asiatica. Different colors are used to highlight distinct gene functional groups. Indication for SSC, LSC and IR region are present. The center is a field photo of S. asiatica.
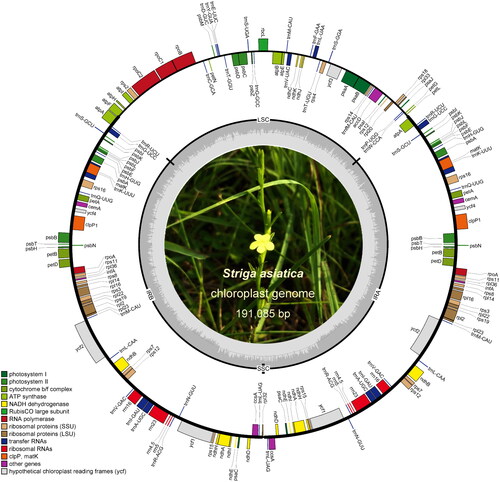
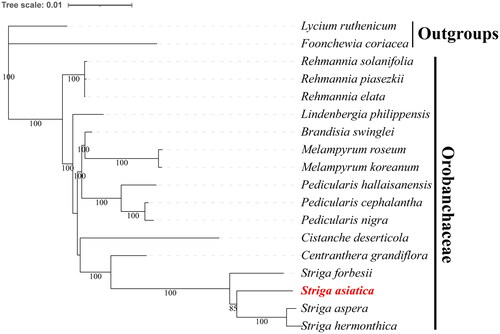
Figure 3. The Maximum likelihood (ML) phylogenetic tree is based on chloroplast genome of S. aisatica and other 17 species. The best-fit model is chosen as TVM + F + I + G4. Bootstrap values are based on 1000 replicates. The numbers on branches are bootstrap support values. Striga asiatica is highlighted in red and bold. The following sequences were used: Striga hermonthica MF780874.1 (Frailey et al. Citation2018), Striga forbesii MF780873.1 (Frailey et al. Citation2018), Striga aspera MF780872.1 (Frailey et al. Citation2018), Striga asiatica ON652844.1 (this study), Rehmannia elata KX636161.1 (Zeng et al. Citation2017), Rehmannia piasezkii KX636160.1 (Zeng et al. Citation2017), Rehmannia solanifolia KX636159.1 (Zeng et al. Citation2017), Pedicularis cephalantha OL606628.1 (Wang et al. Citation2022), Pedicularis nigra OL544940 (Wang et al. Citation2022), Pedicularis hallaisanensis MG770330 (Cho et al. Citation2018), Melampyrum koreanum MW463054 (Jin et al. Citation2021), Melampyrum roseum MN075942 (Yong-Chao et al. Citation2019), Lindenbergia philippensis HG530133.1 (Wicke et al. Citation2013), Brandisia swinglei MK381315.1 (Xia et al. Citation2019), Centranthera grandiflora MW262988 (Zheng and Li Citation2021), Cistanche deserticola KC128846.1 (Li et al. Citation2013), Lycium ruthenicum MK994503.1 (Wang et al. Citation2019), Foonchewia coriacea MT942688 (Zhang et al. Citation2021).
Results and discussion
The complete chloroplast genome of S. asiatica was obtained, with a length of 191,085 bp and a GC content of 37.86%. The genome is composed of a large single-copy region (LSC) of 51,406 bp, a small single-copy region (SSC) of 273 bp, as well as two copies of the inverted-repeat regions (IRa and IRb) of 69,703 bp. The IR region expansion, which resulted in the enlargement of the chloroplast genome, occurred in both the LSC and SSC regions. This type of IRs increases, also observed in other Striga chloroplast genomes, may be unique to particular lineages (Frailey et al. Citation2018). A total of 174 genes were annotated, comprising 122 protein-coding genes (PCGs), 8 ribosomal RNA (rRNA) genes, and 44 transfer RNA (tRNA) genes. As a result of the expansion of IR regions, 46 genes have duplicated copies. Numerous ndh genes were detected as pseudogenized, including ndhA, D, E, H, I, and K, which lacked a 5′-terminal start codon. Moreover, despite possessing complete open reading frames, ndhB, C, and J had significantly shorter gene lengths than their respective homologs. In addition, ndhF and ndhG, were identified as missing genes. The phenomenon of ndh gene loss and pseudogenization had been observed extensively in other parasitic plants (Xu et al. Citation2020; Li et al. Citation2021).
The phylogenetic tree reveals that all Striga species constitute a clade, and S. asiatica is closely related to Striga hermonthica and Striga sepera with the bootstrap value of 100 (). The congruity observed between the phylogenetic outcomes of this investigation and those derived by Frailey et al. (Citation2018) and Zeng et al. (Citation2017) reinforces the current understanding of the evolutionary relationships among Striga species, and thereby advances our comprehension of the genetic diversity and evolutionary history of these parasitic plants.
Conclusion
In conclusion, the sequencing and analysis of the complete chloroplast genome of Striga asiatica using next-generation sequencing technology has provided valuable information about its genetic features and evolutionary relationships with other plants in the Orobanchaceae family, which can contribute to a better understanding of the parasitic mechanisms, molecular markers, and evolutionary patterns of the Striga genus.
Ethics approval and consent to participate
The data collection of plants was carried out with the permission of Yulin Normal University and complied with local (Yulin, Guangxin, China) legislation.
Author contribution
X. H. and Q. L. were involved in the conception and design; K. C. and L. L. contributed the sample collection; E. L. and R. B. performed the analysis and interpretation of the data; X. H. and E. L. contributed the drafting of the paper; Q. L. and X. H. revised it critically for intellectual content. All authors were involved in the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (638.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov under the accession no. ON652844.1. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA892165, SRR22000393, and SAMN31370639, respectively.
Additional information
Funding
References
- Cho W-B, Lee D-H, Choi I-S, Lee J-H. 2018. The complete chloroplast genome of hemi-parasitic Pedicularis hallaisanensis (Orobanchaceae). Mitochondrial DNA B Resour. 3(1):235–236.
- Cochrane V, Press MC. 1997. Geographical distribution and aspects of the ecology of the hemiparasitic angiosperm Striga asiatica (L.) Kuntze: a herbarium study. J Trop Ecol. 13(3):371–380.
- Frailey DC, Chaluvadi SR, Vaughn JN, Coatney CG, Bennetzen JL. 2018. Gene loss and genome rearrangement in the plastids of five Hemiparasites in the family Orobanchaceae. BMC Plant Biol. 18(1):1–12.
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3. 1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64.
- Jin D-P, Kim J-S, Ku Y-B, Lim CE. 2021. The complete chloroplast genome sequence of Melampyrum koreanum (Orobanchaceae), an endemic and hemi-parasitic herb in Korea. Mitochondrial DNA B Resour. 6(11):3122–3124.
- Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241.
- Kakpure M, Rothe S. 2012. Qualitative phytochemical screening of Indian witchweed: Striga asiatica (L.) O. Ktze – an unexplored medicinal parasitic plant. J Exp Sci. 3(3):28–31.
- Li X, Yang J-B, Wang H, Song Y, Corlett RT, Yao X, Li D-Z, Yu W-B. 2021. Plastid NDH pseudogenization and gene loss in a recently derived lineage from the largest hemiparasitic plant genus Pedicularis (Orobanchaceae). Plant Cell Physiol. 62(6):971–984.
- Li X, Zhang T-C, Qiao Q, Ren Z, Zhao J, Yonezawa T, Hasegawa M, Crabbe MJC, Li J, Zhong Y. 2013. Complete chloroplast genome sequence of holoparasite Cistanche deserticola (Orobanchaceae) reveals gene loss and horizontal gene transfer from its host Haloxylon ammodendron (Chenopodiaceae). PLoS One. 8(3):e58747.
- Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C. 2023. CPGView: a package for visualizing detailed chloroplast genome structures. Mol Ecol Resour. 23(3):694–704.
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Rozewicki J, Li S, Amada KM, Standley DM, Katoh K. 2019. MAFFT-DASH: integrated protein sequence and structural alignment. Nucleic Acids Res. 47(W1):W5–W10.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73.
- Spallek T, Mutuku M, Shirasu K. 2013. The genus Striga: a witch profile. Mol. Plant Pathol. 14(9):861–869.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- Wang C, Jia G, Zhu L, Tian F. 2019. The complete chloroplast genome sequence of Lycium ruthenicum (Solanaceae), a traditional medicinal plant in China. Mitochondrial DNA B Resour. 4(2):2495–2496.
- Wang W-J, Liu R, Wu Y, Wang H, Yu W-B. 2022. The complete chloroplast genomes of two Pedicularis species (Orobanchaceae) from Southwest China. Mitochondrial DNA B Resour. 7(6):971–973.
- Wicke S, Müller KF, De Pamphilis CW, Quandt D, Wickett NJ, Zhang Y, Renner SS, Schneeweiss GM. 2013. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell. 25(10):3711–3725.
- Xia Z, Wen J, Gao Z. 2019. Does the enigmatic Wightia belong to Paulowniaceae (Lamiales)? Front Plant Sci. 10:528.
- Xu W, Chen H, Tian L, Jiang M, Yang Q, Wang L, Ahmad B, Huang L. 2020. Extensive gene loss in the plastome of holoparasitic plant Cistanche tubulosa (Orobanchaceae). Mitochondrial DNA B Resour. 5(3):2679–2681.
- Yong-Chao L, Zhou X-R, Yang J, Fu Q-Y. 2019. Characterization of the complete chloroplast genome of Melampyrum roseum. Mitochondrial DNA B Resour. 4(2):3268–3269.
- Yoshida S, Kim S, Wafula EK, Tanskanen J, Kim Y-M, Honaas L, Yang Z, Spallek T, Conn CE, Ichihashi Y, et al. 2019. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr Biol. 29(18):3041–3052.e4. e3044.
- Zeng S, Zhou T, Han K, Yang Y, Zhao J, Liu Z-L. 2017. The complete chloroplast genome sequences of six Rehmannia species. Genes (Basel). 8(3):103.
- Zhang Y, Chen S, Xu X, Wang R. 2021. The complete chloroplast genome of Foonchewia coriacea (Rubioideae: Rubiaceae): a monotypic species endemic to Guangdong, China. Mitochondrial DNA B Resour. 6(1):156–157.
- Zheng L-P, Li L-J. 2021. Characterization of the complete chloroplast genome of Centranthera grandiflora Benth (Orobanchaceae), an important species of medicinal herb. Mitochondrial DNA B Resour. 6(6):1784–1785.
