Abstract
Ascaridia galli (Nematoda: Ascaridiidae), infecting mainly the small intestine of chickens, is one of the most common nematodes in poultry worldwide. The complete mitochondrial genome sequence of A. galli was 13,981 bp in total length with 36 coding genes, namely, 12 protein-coding genes (PCGs), two ribosomal RNAs, and 22 transfer RNAs. All PCGs were transcribed in one direction. Phylogenetic analysis of the mitogenome of A. galli would further contribute to resolving its phylogenetic position and offer novel perspectives on phylogenetic studies of A. galli.
Introduction
Ascaridia galli (Schrank, 1788) (Nematoda: Ascaridiidae), is the largest avian nematode in the Ascaridiidae family and one of the most common nematodes in poultry worldwide, infecting mainly the small intestine of chickens (Urbanowicz et al. Citation2018). A. galli can lead to anorexia, weight loss, bleeding of the intestinal mucosa, alteration of hormone levels, and even death for the host (Permin et al. Citation2006), thus negatively affecting the economic development of the livestock and poultry industries. In cases of genetic isolation or interpopulation hybridization, the structure of mitochondrial DNA (mtDNA) tends to remain unchanged compared to that of autosomally inherited DNA, and therefore, mtDNA is the main source of markers for population inheritance and evolutionary studies (Shao and Barker Citation2007; Muchadeyi et al. Citation2008). This study was undertaken to better understand the biome of A. galli and to provide new insights.
Materials and methods
Parasite specimens were collected from the small intestine of a domestic chicken in Shexian County (N29°51′, E118°24′), Huangshan City, Anhui Province, China. After collection, the nematodes were repeatedly rinsed with 0.9% normal saline and stored in 70% alcohol solution. They were identified as female A. galli according to morphological and molecular characteristics (Tian-yu et al. Citation2020). A specimen was deposited at the Department of Parasitology, Wannan Medical College, Anhui Province, China (Jinhong Zhao, [email protected]), under voucher number WNMC-I-108.
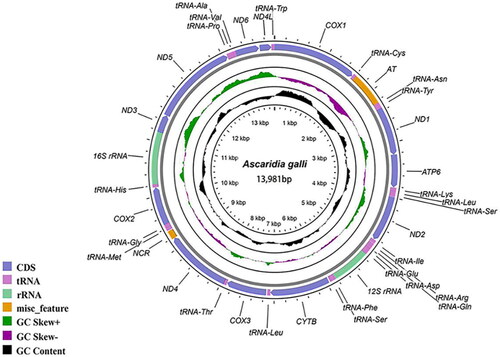
The A. galli female body is yellowish white and cylindrical, with a straight tail tip and a total length of 5.9 cm in the study (). Total genomic DNA was extracted from a single A. galli sample using the TIANamp Genomic DNA Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions. For total genomic DNA sequencing, Sanger sequencing was performed by GENERAL BIOL Co. (Chuzhou, China). The complete genome sequence was archived in the GenBank DNA database under accession no. OQ286042. The mitochondrial genome map of A. galli was constructed using the CGView Server (Grant and Stothard Citation2008) (http://cgview.ca).
Figure 1. Reference image of Ascaridia galli collected from Shexian County, Anhui Province, China. The photo was taken by Yujun Shuai.

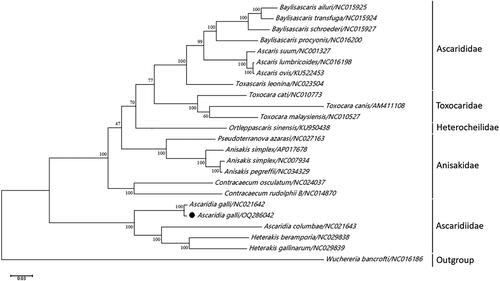
To further investigate the phylogenetic relationships of A. gall, a maximum-likelihood (ML) tree was established by 12 protein-coding genes (PCGs) of 24 nematode mitochondrial genomes downloaded from GenBank. Phylogenetic analyses were conducted using ML methods with Wuchereria bancrofti (GenBank accession no. NC016186) as the outgroup.
Results
The mtDNA size of A. galli (GenBank accession no. OQ286042) was 13,981 bp, with an A + T bias of 76.10% and 12 PCGs, 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (rRNAs), and two noncoding regions (NCRs) (). Similar to the findings in most other nematodes, the atp8 gene is not present in the mt genome of A. galli, and the transcription of all mitochondrial genes is unidirectional (Liu et al. Citation2013). Twelve PCGs, except cytb, cox3, and nad4L, were inferred to use the incomplete stop codon ‘T’, and the remaining nine genes (cox1, nad1, atp6, nad2, nad4, cox2, nad3, nad5, and nad6) were predicted to use the typical stop codon ‘TAG’ or ‘TAA’. Twenty-two tRNAs ranged in length from 51 bp (tRNA-ser) to 63 bp (tRNA-Met). For the two rRNAs, the small subunit rRNA (rrnS) between tRNA-Glu and tRNA-Ser had a length of 700 bp, and the large subunit rRNA (rrnL) located between tRNA-His and nad3 had a length of 955 bp. Regarding the two NCRs, the NC1 region (also regarded as the AT-rich region, 615 bp) and NC2 (158 bp) were located between tRNA-Cys and tRNA-Asn and between nad4 and tRNA-Met, respectively.
The ML phylogenetic tree showed that all the nematodes of the order Ascaridida belonged to five families: Anisakidae, Ascarididae, Toxocaridae, Heterocheilidae, and Ascaridiidae. A. galli was highly homologous to Ascaridia galli (GenBank accession no. NC021642) in GenBank, forming a monophyletic group belonging to the family Ascaridiidae. Moreover, we found that the results of phylogenetic analyses obtained using Bayesian inference models were similar. The chicken roundworm examined in this study had high bootstrap values for inclusion in Ascaridiidae ().
Discussion and conclusions
In the present study, the complete mitogenome of A. galli was assembled and analyzed. The mitochondrial genome of A. galli is 13,981 bp in length and expresses high AT bias. Phylogenetic analysis was also performed. We expect that these data will be conducive to providing new references and insight for studies on the phylogenetics and genetics of A. galli.
Author contributions
All authors contributed to the research conception and design. Materials preparation, data collection, and analysis were completed by Yujun Shuai, Qiqi Xue, Minghui Zou, and Jinhong Zhao. The first draft of the manuscript was written by Yujun Shuai, and all the authors commented on previous manuscript versions, approved the final manuscript, and agreed to be responsible for all aspects of the work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. This study did not require ethical approval or permission.
Supplemental Material
Download MS Word (17.2 KB)Supplemental Material
Download MS Word (21.2 KB)Supplemental Material
Download MS Word (110.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/nuccore/OQ286042.
Additional information
Funding
References
- Grant JR, Stothard P. 2008. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 36:W181–W184. doi: 10.1093/nar/gkn179.
- Liu G-H, Shao R, Li J-Y, Zhou D-H, Li H, Zhu X-Q. 2013. The complete mitochondrial genomes of three parasitic nematodes of birds: a unique gene order and insights into nematode phylogeny. BMC Genomics. 14(1):414. doi: 10.1186/1471-2164-14-414.
- Muchadeyi F, Eding H, Simianer H, Wollny C, Groeneveld E, Weigend S. 2008. Mitochondrial DNA D‐loop sequences suggest a Southeast Asian and Indian origin of Zimbabwean village chickens. Anim Genet. 39(6):615–622. doi: 10.1111/j.1365-2052.2008.01785.x.
- Permin A, Christensen J, Bisgaard M. 2006. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet Scand. 47(1):43–54. doi: 10.1186/1751-0147-47-43.
- Shao R, Barker S. 2007. Mitochondrial genomes of parasitic arthropods: implications for studies of population genetics and evolution. Parasitology. 134(Pt 2):153–167. doi: 10.1017/S0031182006001429.
- Tian-Yu R, Xi S, Zhi-Wei Z, Wei X, Jin-Hong Z. 2020. Morphological and molecular characteristics of Ascaridia galli isolates from Huangshan area. J Trop Dis Parasitol. 18:87.
- Urbanowicz J, Gaweł A, Bobrek K. 2018. Ascaridia galli isolates with ITS1-5.8 rRNA-ITS2 fragment homologous to Ascaridia columbae. Acta Parasitol. 63(3):640–644. doi: 10.1515/ap-2018-0073.