Cholangiocarcinomas (CCAs) include a heterogeneous group of hepatobiliary tumors accounting for approximately the 10–15% of primary liver cancers [Citation1]. Unfortunately, most of these tumors are diagnosed at an advanced stage, and more than ten years after the publication of the landmark phase III ABC-02 trial establishing gemcitabine-cisplatin as first-line standard for metastatic CCA, the prognosis of this patient population remains grim [Citation2]. In fact, most of patients treated with front-line treatment fail to achieve a response or responses are short lived [Citation3]. Recent years have witnessed the advent of molecular profiling in this setting, and new techniques and technologies have led to the identification of a variety of molecular alterations in CCA [Citation4]. Over the last decade, several potentially actionable genetic aberrations have been highlighted, and precision oncology approaches have been evaluated and are under investigation in these hepatobiliary malignancies (). A large number of anticancer agents are currently in development, including Fibroblast Growth Factor Receptor (FGFR) 2, Isocitrate Dehydrogenase 1 (IDH-1), and BRAF inhibitors [Citation5]. FGFR2 aberrations have been reported in approximately the 20% of intrahepatic cholangiocarcinomas (iCCAs), with these alterations highlighted as most common in female patients and young adults [Citation6]. Of note, FGFR2 aberrations are mainly represented by fusions, while mutations are detected only in a minority of patients. As regards IDH-1, missense mutations are the most frequent aberrations, involving a single residue in the active site of the enzyme [Citation7]; typically, IDH-1 missense mutations have been reported to be more common in small duct type iCCAs and in poorly differentiated or undifferentiated forms [Citation8]. Several other molecular aberrations have been observed in CCA patients, including BRCA mutations, NTRK fusions, and BRAF V600E mutations [Citation9,Citation10].
Figure 1. Schematic figure reporting the main signaling pathways and selected targeted therapies currently under assessment in cholangiocarcinoma patients.
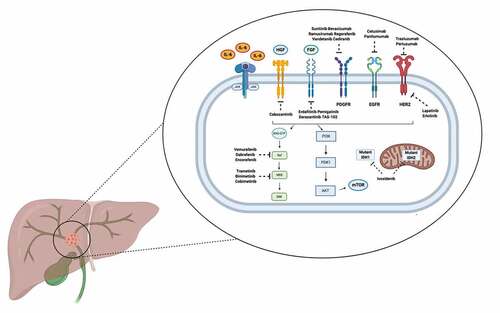
Nonetheless, several questions remain unanswered, and the adoption of precision oncology in CCA patients remains still far from everyday clinical practice, with some important exceptions. Among current obstacles, a crucial point to highlight is the limited access to anticancer treatments. For example, although the final overall survival (OS) analysis of the ClarIDHy phase III trial has been presented and on 25 August 2021 the FDA approved ivosidenib use in IDH-1 mutant iCCA, the IDH-1 inhibitor is not available in several countries, according to different choices by the regulatory agencies [Citation11].
Second, biopsy samples are often inadequate for molecular profiling since tissue sampling has reported low sensitivity in the diagnosis of malignant biliary strictures; in fact, the highly desmoplastic nature of CCA severely limits the accuracy of pathological and cytological methodologies. On the basis of these premises, it is urgent to develop in this scenario novel strategies aimed at anticipating the diagnosis by detecting CCA at an early, resectable stage, as well as to obtain adequate material to perform genomic analysis. Among these strategies, liquid biopsy may represent a fundamental tool, although the use of this technique is still limited and has to be implemented in the near future [Citation12].
A long-standing issue is also represented by the genomic diversity of CCAs that also mirrors anatomical, epidemiological, and therapeutical differences. In fact, it is currently well known that these malignancies should not be considered as a single entity but a group of tumors with heterogeneous features. However, several trials still group together different anatomical CCA subgroups, such as iCCA, perihilar CCA, and distal CCA, something that represents a ‘historical’ issue in clinical trial design in these hepatobiliary malignancies. Stratification of patients according to CCA subgroup should always be present in studies on these tumors, also considering that distinct subgroups are associated to different molecular features, clinical outcomes and prognosis.
In our opinion, another landmark topic in this setting is represented by clinical trial enrollment, something that remains a priority in CCA due to the difficulties encountered in enrolling patients with these rare hepatobiliary tumors. For example, the ClarIDHy and the FIGHT-202 trials on ivosidenib and pemigatinib, respectively, enrolled more than a hundred CCA patients, requiring important efforts in terms of screening and enrollment [Citation13–15]. Similarly, the investigators of the phase II, single-arm, ROAR trial evaluating dabrafenib plus trametinib in BRAF V600E-mutated CCAs, had to prescreen 626 subjects to enroll 43 CCA patients, a figure that highlights the difficulties in completing such a trial [Citation16]. Conducting clinical studies in rare CCA subgroups with genetic aberrations remains a compelling challenge, and international collaborations are of pivotal importance in this setting. This is even more important in CCA patients whose disease has progressed following first-line or second-line treatment, where genomic testing and enrollment in biomarker-driven clinical studies investigating novel targeted therapies and combination treatments is fundamental. In addition, promoting basic and translational research in this setting remains a priority, and several novel research avenues are quickly emerging, including tumor microenvironment [Citation17].
Precision oncology is an exciting area in hepatobiliary tumors, and we have recently witnessed unprecedented advances in CCA management, with the fast development of novel agents and therapeutic options [Citation18]. In our opinion, in 2021, next-generation sequencing should represent one of the first steps in a newly diagnosed CCA patient, regardless of disease stage. Further large-cohort prospective trials and investigations are mandatory in order to better define new paradigms of treatment in these heterogeneous, aggressive malignancies with many unanswered questions.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma ‐ evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95‐111.
- Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281.
- Kelley RK, Bridgewater J, Gores GJ, et al. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353–363.
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588.
- Forner A, Vidili G, Rengo M, et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39:98–107.
- Rizzo A, Ricci AD, Brandi G. Pemigatinib: hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun. 2021;27:100337. Epub 2021 Feb 18. PMID: 33611090.
- Ntanasis-Stathopoulos I, Tsilimigras DI, Gavriatopoulou M, et al. Cholangiocarcinoma: investigations into pathway-targeted therapies. Expert Rev Anticancer Ther. 2020 Sep;20(9):765–773. Epub 2020 Aug 16. PMID: 32757962.
- Zhang W, Zhou H, Wang Y, et al. Systemic treatment of advanced or recurrent biliary tract cancer. Biosci Trends. 2020 Nov 4;14(5):328–341. Epub 2020 Aug 24. PMID: 32830166.
- Mahipal A, Kommalapati A, Tella SH, et al. Novel targeted treatment options for advanced cholangiocarcinoma. Expert Opin Investig Drugs. 2018 Sep;27(9):709–720. Epub 2018 Aug 30. PMID: 30124336.
- Rizzo A, Frega G, Ricci AD, et al. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: a systematic review and meta-analysis. In Vivo. 2020 Mar-Apr;34(2):479–488. PMID: 32111744; PMCID: PMC7157865.
- Gervaso L, Pellicori S, Fazio N. Ivosidenib for advanced IDH1-mutant cholangiocarcinoma. Lancet Oncol. 2020 Aug;21(8):e370. PMID: 32758465.
- Ettrich TJ, Schwerdel D, Dolnik A, et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep. 2019 Sep 13;9(1):13261. PMID: 31519967; PMCID: PMC6744511.
- Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020 Jun;21(6):796–807. Epub 2020 May 13. Erratum in: Lancet Oncol. 2020 Oct;21(10):e462.PMID: 32416072; PMCID: PMC7523268.
- Zhu AW, Macarulla T, Javle M, et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. J Clin Oncol. 2021;39(3_suppl):266.
- Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684.
- Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020 Sep;21(9):1234–1243. Epub 2020 Aug 17. PMID: 32818466.
- Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019 May;39(Suppl 1):63–78. PMID: 30907492.
- Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020 Aug;73(2):315–327. Epub 2020 Mar 12. PMID: 32173382; PMCID: PMC8418904.
