Abstract
Endoreduplication is a modified form of mitotic cycle in which repeated rounds of DNA replication occur without chromosome segregation and cell division, leading to formation of larger and thicker chromosomes called polytenics. In the present study, spontaneous formation of polytene chromosomes in the generative nuclei of pollen grains of Amaryllidaceae, and presumably in angiosperms, was described for the first time. For this purpose, microspores and mature pollens of the endangered species Pancratium maritimum L. were examined by light and transmission electron microscopy with a special reference to the observation of polytenic nuclei. Polytene chromosomes were detected in most of the generative cells of young bicellular pollen grains and mature pollen grains found in some anther locules, but they were rare or absent in the other anther locules. Polytenization of chromosomes did not follow the same pattern in all of the pollen grains. Polytene structures found in the developing pollen grains of P. maritimum exhibited variation from a diffuse to a condensed state and from reticulate to cable-like structures with different degrees of bonding. In addition to cryptic polyteny, cable-like polytene chromosomes with bands resembling classic polytene chromosomes were also detected. However, some nuclei did not exhibit polytenic structure clearly. Morphological changes in polytene chromosomes that are caused by the formation of functional structures such as DNA amplification, puffing and looping were also recognized. Other reasons for morphological differences of polytene chromosomes are discussed in detail.
Introduction
Presence of different ploidy levels within an organism is described by the term endopolyploidy. Endopolyploidy is caused by two different nuclear cycles called endoreduplication and endomitosis. Nagl (Citation1978, Citation1981, Citation1987) has used the term endocycle as a general description of the both endoreduplication and endomitosis. Although these two terms (endoreduplication and endomitosis) of the nuclear cycles are often used indiscriminately in the literature, d’Amato (Citation1984) has proposed the term endomitotic for the endocycle that produces polyploid nuclei and endoreduplication for that which generates polytene nuclei (Carvalheira Citation2000). On the other hand, all types of endopolyploidy are not caused by endocycle, as in binucleate and multinucleate cells (Barow Citation2006).
Endomitosis is similar to normal mitosis in terms of the structural changes that occur during nuclear cycles. The initial steps of mitosis up to chromosome separation proceed normally inside the nucleus with an intact nuclear membrane. It starts with the normal prophase (endoprophase), continues with the condensation of the chromosomes (endometaphase), after which sister chromatids separate from each other but do not move to the opposite poles (endoanaphase). Therefore, instead of entering the telophase stage, chromosomes decondense and return to their interphase state. As a result of this, cytokinesis does not take place and chromosome number doubles in each cycle, leading to the formation of polyploid cells.
The endoreduplication cycle, which results in the formation of polytenic cells, differs from endomitosis and normal mitosis in many aspects. In the normal mitotic cycle of a diploid cell, there are four phases: G1, S, G2 and M phases. However, in the interphase stage of an endoreduplication cycle, the G2 phase of the mitotic cycle is omitted. Therefore, in this shortened nuclear cycle, the DNA content of chromatin is duplicated during the S phase but instead of entering into G2 phase, the nucleus returns to G1 phase and starts a new cycle of chromatin duplication. These repeated rounds of DNA replication, without chromatid separation and cell division, lead to the formation of larger, thicker and multi-stranded polytene chromosomes.
In summary, incomplete mitosis and the presence of multiple doubled DNA in the cell are the common traits of both endomitosis and endoreduplication. However, in contrast to endomitosis, during the endoreduplication cycle, duplicated chromatids do not separate and remain paired to each other to different degrees. Therefore, the chromosome number does not increase during endoreduplication.
Polytene chromosomes allow the direct microscopic observation of chromosome organization, heterochromatin, gene location, puffing (differential gene activity), DNA under-replication and amplification. Therefore, they have been very important objects for cell biologists since their discovery, and most of the substance of genetics, cytogenetics and cell biology is based on the study of polytene chromosomes (Nagl Citation1981). Polytene chromosomes have been used for the analysis of numerous features of interphase chromosome organization and the genome as a whole. Furthermore, polytene chromosomes permit the analysis of gene activity and its control directly at the gene level (Ashburner Citation1970; Zhimulev and Koryakov Citation2009).
Polytene chromosomes have been reported in dipteran larval and adult tissues, in protozoa, in mammals and in angiosperms (Pearson Citation1974; Nagl Citation1981). In plants, polyteny occurs frequently in specialized cells and tissues with high metabolic activity and in tissues that require rapid development. Storage tissues of angiosperms such as immature seed tissue (endosperm) also have polytenic nuclei. Until recently, polytene chromosomes have been reported in ovary tissues that nourish the developing embryo such as antipodes (Nagl Citation1981; Petrova, Solovyanova, and Chentsov Citation1985), synergids (Hasischka-Jenschke Citation1957; Nadirashvili, Gvaladze, and Akhalkatsi Citation2006), embryo suspensor cells (Nagl Citation1974; Freed and Grant Citation1976;) and endosperm (Nagl Citation1981; Rajya Lakshmi et al. Citation2001). Moreover, numerous papers deal with the occurrence of the polytene chromosomes in the anther tapetum cells of many plant species including Vigna unguiculata (L.)Walp. (Guerra and Carvalheira Citation1994; Kumar and Verma Citation2011) and some Phaseolus species (Carvalheira and Guerra Citation1994). Polytene chromosomes were also studied in the anther hairs of Bryonia dioica Jacq. (Nagl Citation1981) and the glandular hairs of Salvia horminum L. (Gostev and Asker Citation1978).
In Amaryllidaceae, polytene chromosomes have been reported only in a few ovary tissues including the preferred caesura antipodes – Clivia miniata (Lindl.) Bosse (Nagl Citation1981); Scilla bifolia L. (Nagl Citation1976) –,and embryo suspensor cells – Gagea lutea (L.) Ker Gawl. (Nagl Citation1981). To the best of my knowledge, there is no report about the spontaneous formation of polytenic nuclei in the pollen grains of Amaryllidaceae and presumably in angiosperms. Moroever, the literature survey for the study revealed that most of the studies on polyteny have been performed on somatic cells. However, to elucidate the role of endoreduplication in genetic processes and evolution, it is necessary to investigate the occurrence of polyteny in generative cells. Hence, in this study, the spontaneous formation of polytene chromosomes in the developing microspores and mature pollen grains has been researched using endangered plant species Pancratium maritimum L., as a model. The primary purpose of this article is to make a contribution to the understanding of polytenics in the generative cells of angiosperm pollen grains by studying the presence and type of polytene chromosomes found in the developing and mature pollen grains of the studied plant.
Pancratium L. is a small genus in the family Amaryllidaceae including about 21 species of perennial, herbaceous and bulbous plants distributed in the Old World (Asia, Africa and Europe) (Mabberley Citation2008; De Castro et al. Citation2012). In the Turkish flora, Pancratium L. is represented by only one species, Pancratium maritimum, which is widely distributed along the Black Sea and the Mediterranean coasts of Turkey (Mill Citation1984). As in the other coastlines of the Mediterranean and the Black Sea, P. maritimum is threatened with extinction in its original habitat in Turkey and therefore classified as rare and endangered (EN) in the red data book of Turkish plants (Ekim et al. Citation2000). The flowering period of P. maritimum in Turkey occurs from late June to early September. The species shows phenotypical variation, which is expressed by the differences in the length and wideness of leaf blade and the dimension of bulb that is buried 30–80 cm deep in the sand. The diploid chromosome number of P. maritimum growing naturally in the Central Black Sea region of Turkey (A6 grid square) was reported as 22 by Şenel, Özkan, and Kandemir (Citation2002).
Material and methods
Fresh flower buds and mature anthers of P. maritimum plants growing naturally in the Black Sea coasts of Tekirdağ A1 (E), European Turkey were collected at different time intervals (10:00–10:30 am and 14:00–17:00 pm) in August 2014. About 35 anthers at various stages of development were collected from different P. maritimum specimens found in the same location. Following dissection from the buds, anthers at different developmental stages were pre-fixed immediately in 3% glutaraldehyde in Sorensen’s buffer (pH 7.4) for 24 h at 4°C and rinsed in Sorensen’s buffer three times for 15 min each. After post-fixation with 1% osmium tetroxide in the same buffer (pH 7.4) for 2 h at room temperature, the anthers were dehydrated in a series of increasing alcohol in water and were embedded in Epon according to the usual method (Craig, Frojola, and Greider Citation1962).
Light microscopy
For light microscopy studies, 1-μm transverse sections were cut using a Leica Em UC6 ultramicrotome. Semi-thin sections were stained with toluidine blue (O’Brien, Feder, and McCully Citation1964) for general histological observations to highlight cell components and especially chromosomes. Toluidine blue stains nucleic acids (acidic polyanionic groups) blue and polysaccharides purple.
Transmission electron microscopy
For transmission electron microscopy studies, ultra-thin sections (60–100 nm) were cut from Epon-embedded material with a Leica Em UC6 ultramicrotome and stained with uranyl acetate and lead citrate. Electron micrographs were taken with an FEI Tecnai G2 (120 kv) transmission electron microscope.
Results
Polytenic nuclei in bicellular pollen grains
Cytological examination of the semi-thin sections of some anther loci of P. maritimum showed that there were differences in the size and appearance of young bicellular pollen grains found in the same anther locule. Also, there were striking morphological peculiarities in their nuclei and chromosomes, compared with each other and with nuclei showing normal development (Figure ). Detailed investigation of these nuclei revealed the presence of polyteny in most of the generative cells of these pollen grains. Furthermore, it was determined that there was more than one type of polytene structure in the generative nuclei of bicellular pollen grains found in the anther locules (Figures ). Polytene structures varied from diffuse to condensed state and from reticulate to cable-like structures with different degrees of bonding. In condensed state, bundles of sister chromatids were detected and polytene chromosomes were seen, as either loosely attached cable-like structures (Figure d) or tightly bonded structures (Figure a). In some pollen grains, polytene chromosomes could be identified easily because of their similarity to the classic polytene chromosomes found in the salivary glands of diptera (Figure ). For example, polytene chromosomes with characteristically banding structures were recognized (Figures c, d; d). However, bands were not present throughout the polytene chromosomes.
Figure 1. Transverse sections of different anther locules of Pancratium maritimum showing microspores and immature bicellular pollen grains (stained with toluidine blue). (a) General appearance of microspores found in the anther locule. Note the different sizes and morphology of the microspores. (b–d) Pollen grains in anther locules having generative nuclei in different morphologies. AL, anther locule; AW, anther wall
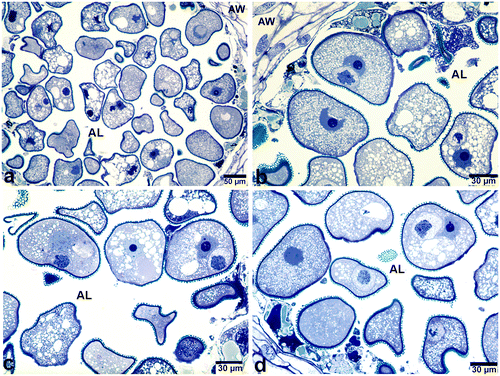
Figure 2. Polytene chromosomes in the generative cell nuclei of bicellular pollen grains stained with toluidine blue (a, b) Easily recognizable polytene chromosome with loop (arrow) and puffing. (c, d) Polytene nuclei in the form of cable-like structures having light and dark bands (arrows). (e, f) Polytene nuclei with loosely attached chromatids. Note the vacuoles found in the nucleolus of the vegetative nucleus in (f). AW, anther wall; Gc, generative cell; V, nucleolar vacuole; Vn, vegetative nucleus.
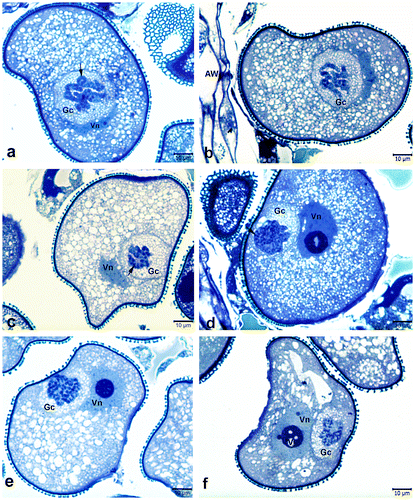
Figure 3. Details of polytenic generative nuclei at different duplication levels. (a) Highly condensed polytenic nuclei including tightly bonded cable like structures. (b, c) Polytenic nuclei in the form of lumps of chromatin (arrows). (d, e) Polytenic nuclei at intermediate stage between chromosomes with multiple chromatids and polytene chromosomes. Note the banded polytene chromosomes (arrows). (f–h) Polytenic nuclei containing chromocentres (long arrows) associated with chromatin bundles. Short arrow in (f) shows the polytenic nucleus in the transitional stage from bundles of chromatids to polytene chromosomes. Gc, generative cell; Vn, vegetative nucleus; Ta, remnants of tapetum. Scale bar 10 μm.
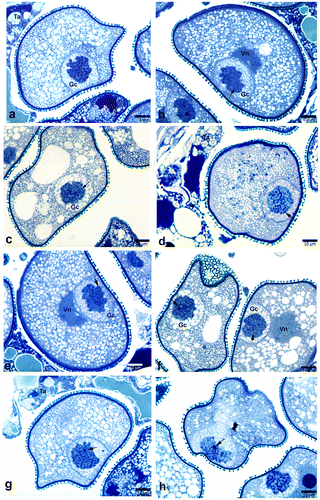
Figure 4. Polytene chromosomes in the generative cell of Pancratium maritimum pollen involving DNA under-replication, DNA amplification and Puffing. (a) Polytenic structure exhibiting DNA amplification. Note that there is degradation in one part of amplified region. (b, e, f) Polytene chromosomes exhibiting puffing (arrow). (c, d) Polytenic chromosome involving DNA under-replication. Note the irregularly shaped vegetative nucleus (arrow) in (d). AW, anther wall; Gc, generative cell; Vn, vegetative nucleus; Ta, remnants of tapetum.
On the other hand, in some of the generative nuclei, polytenic structures were in the form of lumps of chromatin (Figure b, c), it was therefore difficult to identify the polytenic features. Moreover, nuclei in a transitional stage from the chromosomes with multiple chromatids to the polytene chromosomes were also observed (Figures d, e).
In the diffuse state of the polytenic structure, chromocentres associated with chromatin bundles were visible. Puffs and loops were also observed in some polytene chromosomes (Figures a, b; b, e, f). Additionally, polytene chromosomes with DNA amplification and under-replication were also recognized (Figure ). Besides the polytenic generative cells, the pollen grains containing binucleate vegetative cells were also rarely observed.
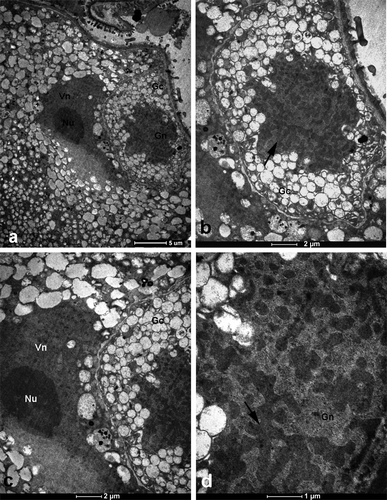
In transmission electron micrographs, generative cells with amoeba-like nuclei containing large blocks of heterochromatin were observed. Cytoplasm of generative cells was very dense and filled with numerous vacuoles (Figure ).
Figure 5. Transmission electron micrographs of section through a single anther locule showing part of bicellular microspore including polytenic generative nucleus. (a) General view of the generative and vegetative nucleus. (b) Detailed view of generative cell shown in (a). Note the polytenic generative nucleus (arrow). (c) Magnified view of vegetative nucleus. (d) Details of generative nucleus. Note the large block of heterochromatin (arrow). Gc, generative cell; Nu, nucleolus; Vn, vegetative nucleus.
Polytenic nuclei in mature pollen grains
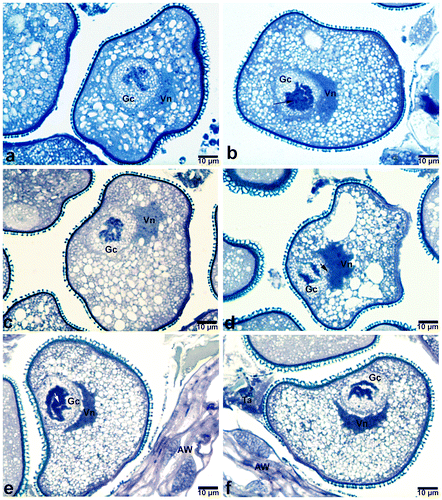
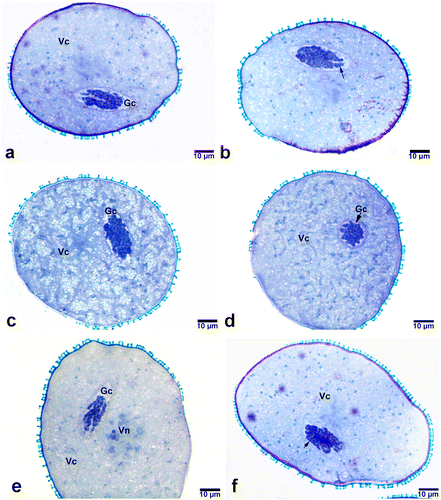
As observed in bicellular young pollen grains, poly-tenic nuclei were also detected in mature pollen grains. Similar to those of bicellular young pollen grains, poly-tenic structures in mature pollen grains also varied from a tightly bonded highly condensed form to a loosely bonded reticulate form (Figure ). Chromatid paring varied regionally along the same polytene chromosome. In the polytenic nuclei where chromatid conjugation is minimal, reticular structure was more distinctive.
Figure 6. Generative nuclei in mature pollens of Pancratium maritimum (at dehiscent stage of anthers). (a, b) Polytenic structures in which chromatid pairing varies regionally. Note the degradation occurring in the polytenic structure in (b) (arrow). (c, d, e) Generative nuclei that do not exhibit polytenic properties. (f) Polytenic nuclei having reticulate structure. Note the loosely attached chromatids (arrow). Gc, generative cell; Vc, vegetative cell; Vn, vegetative nucleus.
Discussion
This study reports the spontaneous development of an endoreduplication cycle in the generative nuclei of angiosperm pollen grains for the first time. According to literature, the occurrence of endoreduplicated generative nucleus in pollen grains seems to be restricted to the induced method of endoreduplication as in Datura innoxia Mill. (Sunderland, Collins, and Dunwell Citation1974) in which microspores were cultured at 28°C for 24 h and artificially stimulated for pollen embryogenesis. Furthermore, endocycle has been observed in mutant (duo pollen1) generative nuclei of Arabidopsis thaliana (L.) Heynh. pollen grains, which complete DNA synthesis, but bypass second pollen mitosis and enter an endocycle during pollen maturation (Durbarry, Vizir, and Twell Citation2005). In the literature survey, we could not identify any report on the spontaneous formation of an endoreduplication cycle in the generative nuclei of pollen grains in Amaryllidaceae or other angiosperm families. Although there is no report on the spontaneous formation of polytene chromosomes in bicellular microspores and pollen grains, the presence of supernumerary nuclei in the generative and vegetative cells of pollen grains has been known for a long time. For example, Poddubnaya-Arnoldi (1976, as quoted in Johri, Ambegaokar and Srivastava Citation2013, 556–558) reported three, four and six nucleate generative cells, and three, four and multinucleate vegetative cells in the developing pollen grains of tetraploid Cucumis melo L.
As indicated in the result section, the appearance of polytenic nuclei and the morphology of polytene chromosomes were different in the same anther locule of the studied plant. Furthermore, some nuclei did not exhibit clearly polytenic properties. These differences may be explained by the presence of different degrees of polyteny in the nuclei of these cells, which depend on how many internal cycles of endoreduplication had occurred at the time of observation. If the degree of polyteny is low in nuclei, it is not possible to observe polytene chromosomes during interphase (Nagl Citation1981). Conversely, d'Amato (1989) has pointed out that in many species of plant and animals, nuclei with high-level polyteny do not contain polytene chromosomes and include, instead, a homogeneous or reticulate structure.
The different categories of morphological differences between the polytene chromosomes of P. maritimum are discussed in detail here.
DNA under-replication, DNA amplification and puffing
Different regions of the same chromosome can be endoreduplicated at a different degree (Nagl Citation1981; Cionini et al. Citation1982). As stated by Nagl (Citation1981), DNA replication in endocycles can follow different pathways and total genome replication does not take place in all cases. Sometimes, a small region of the nuclear DNA is not replicated or is not replicated as often as the main portion of the genome (i.e. DNA under-replication). In a few cases, only a small part of the genome is extra-replicated at the diploid or polyploid level (i.e. DNA amplification).
Similarly, in the present study, all of the DNA fragments in an individual chromatid did not polytenize to the same extent, that is DNA under-replication and amplification were identified. In addition to DNA under-replication and amplification, puffs and loops were also observed on some polytene chromosomes at different positions (Figure ). Therefore, it can be proposed that some of the morphological differences in polytene nuclei have been caused by DNA under-replication, amplification and puffing.
Diffuse and condensed state of polytenic structure
As reported previously in the tapetal cells of some members of the genera Vigna Savi and Phaseolus L. (Guerra and Carvalheira Citation1994), polytene chromosomes of P. maritimum exhibited cycles of diffuse and condensed states. In the diffuse state, only endochromocentres and sometimes the diffuse chromatin were visible. However, in condensed state, the bundled sister chromatids were observed and identified as polytene chromosomes. They showed a variable condensed region and one or two terminal decondensed regions. A polytenic nucleus in transitional stage from diffuse to condensed state was also observed. According to Carvalheira and Guerra (Citation1994), the change from diffuse to condensed state seems to depend mainly on the endoreduplication level, the genetic background and environmental factors. However, Nagl (Citation1978) has identified that the condensed and diffuse structures of chromosomes depend on the environmental conditions; as an example, the extent of chromosome condensation may change under unfavourable conditions such as cool temperature and an exceptionally long day.
Degree of chromatid pairing
Different morphologies of polytene chromosomes may also be attributed to the degree of chromatid attachment, which is determined by many factors such as the physiological state of the cell, the stage of polytene chromosome formation – the number of replication rounds (Zybina and Zybina Citation2011, 227–58) and genetic variation (Ribbert Citation1979). If chromatid attachment is minimal, as is the case in plants, a polyploid nucleus with a reticular structure named as “cryptic polyteny” is formed. However, if homologous chromatid conjugation is maximal, classic polytene chromosomes in the form of cylindrical cables with a distinct banding pattern are formed (Zhimulev and Koryakov Citation2009).
It is interesting to note that, in the current study, in addition to cryptic polyteny, cable-like polytene chromosomes with characteristic banding structures, resembling the classic polytene chromosomes, have been observed. However, bands were not observed throughout the polytene chromosomes, indicating that chromatid attachment is taking place only in some regions of the polytene chromosomes. Another possible explanation for the discontinuity in the banding structure is that the assembly of chromatids to the polytene chromosomes has not been completed, because of an insufficient number of endocycles (i.e. polytenic nuclei are in a transitional stage from the chromosomes with multiple chromatids to the polytene chromosomes due to lack of a sufficient number of chromatid conjugations). Therefore, it is possible that after many successive endocycles, chromatid attachment would be maximum and bands would be observed throughout the polytene chromosome.
Although it had been thought for a long time that plant polytenics do not have distinct bands, there are numerous reports dealing with the banding pattern of plant polytenics. Nagl (Citation1969) reported the formation of banded polytene chromosomes in the embryo suspensor cells of Phaseolus vulgaris that are exposed to low temperature. Later, polytene chromosomes with indistinct banding patterns have been reported in many cereals including wheat (Evers Citation1970; Donovan Citation1979; Chojecki, Bayliss, and Gale Citation1986), corn (Kowles and Phillips Citation1985) and rice (Ramachandran and Raghavan Citation1989). Finally, Rajya Lakshmi et al. (Citation2001) have reported the presence of a clear banding pattern in the polytene chromosomes of pearl millet endosperm nuclei, in both in vivo and in vitro conditions. Today it is admitted that polytene chromosomes in some plants may present a banding pattern. However, as stated by Khanna (Citation2010, 32–9), clear banding patterns in plant polytenics are less frequent than in animals. This can be explained by the fact that in the polytene chromosomes of plants, chromomeres are not lined up exactly in parallel, and as a consequence, the denser chromatin parts do not lie side-by-side, to form a distinctive banding pattern. According to previous investigators (Brady and Clutter Citation1974; Cionini et al. Citation1982; Carvalheira Citation2000), bundled polytene chromosomes of plants may be produced by the advanced endoreduplication cycles, which result in prophase or prophase-like chromosomes that can carry on DNA and RNA synthesis.
Polytenic nuclei in the suspensor cells of some Phaseolus species have been studied extensively by Nagl (Citation1970) and Brady and Clutter (Citation1974). The results of their studies have revealed that, although bundles of chromatin threads with indistinct bands and chromocentres were present in some nuclei, only lumps of chromatin were visible in other nuclei. These results are consistent with the findings of the current study. Additionally, Brady (Citation1973) has reported that in the suspensor tissue of Phaseolus coccineus L., only cells with DNA content higher than 64C presented polytene chromosomes. On the other hand, Nagl (Citation1974) has observed that embryo suspensor cells with 256C showed polytene chromosomes in Phaseolus caffer Haberle ex Steud. and Phaseolus tuberosus Eaton & Wright, but not in Phaseolus lunatus L. and Phaseolus acutifolius A.Gray, suggesting a genomic or genic dependence (Carvalheira and Guerra Citation1994).
Zybina and Zybina (2011, 234) have pointed out three main types of polytene nuclei: (1) nuclei containing classic polytene chromosomes with a characteristic banding structure; (2) nuclei that express polytene features partly as in the suspensor cells of higher plants (Nagl Citation1978, Citation1981); and (3) the nuclei in which the polytene features are not revealed and polyteny may be suggested based on the multifold DNA replication in the absence of mitosis (Brodsky and Uryvaeva Citation1985, 154–161). Indeed, all of these types of polytene nuclei have been observed in the current study.
Suggested functional roles for endoreduplication
Since endoreduplication is very common among angiosperms, numerous studies have been performed to understand its developmental, evolutionary and ecological significance. Although there are many studies on the physiological role of endoreduplication during plant development, studies that address its evolutionary and ecological function have not yet succeeded in explaining the phenomenon clearly. Therefore, the functional role of endoreduplication remains ambiguous.
Until recently, many functions have been proposed for the endoreduplication cycle during plant development. Most of them are related to cell size, cell differentiation and cellular metabolism (Chevalier et al. Citation2011). For example, Comai (Citation2005) has stated that an increase in the amount of DNA and consequently, in cell volume can be advantageous for cells that have a high metabolic rate. Hence, they can respond to developmental needs. Recently, by considering the reports dealing with the role of endoreduplication, Chevalier et al. (Citation2011) have proposed that endoreduplication might contribute to the adaptation of plants to adverse environmental factors by maintaining the growth under stress conditions such as high salt concentration (Ceccarelli et al. Citation2006), water deficit (Cookson, Radziejwoski, and Granier Citation2006) and low temperatures (Barow Citation2006). Chevalier et al. (Citation2011, 161) have also stated that “the extended amplification of nuclear DNA may protect the genome from DNA – damaging conditions such as UV damage or prevent uneven chromosome segregation during mitosis”. According to these authors, endoreduplication is likely to have been selected during evolution for the benefit of plant and organ development.
Pancratium maritimum is an endangered species threatened with extinction. Although this situation is mostly related with human activities, the author thinks that there might be a correlation between the occurrence of endoreduplication cycle and the high risk of extinction of the species. It is possible that some members of this species have developed endoreduplication cycle to adapt unfavorable environmental conditions. According to Scholes and Paige (Citation2015) endoreduplication occurs in many taxa in response to numerous environmental stresses. As suggested by these authors, some members of P. maritimum might have developed endo-reduplication as an adaptive, plastic response to the stresses of the adverse environmental conditions.
In summary, the following conclusions can be derived from the current study and literature survey: (1) polytene chromosomes may develop spontaneously in the generative nuclei of some pollen grains possibly in response to numerous environmental stresses (2) polytene chromosomes in plants can show structural variations from tightly bundled to more loosely bundled and from banded organization to indistinctly banded or unbanded organization.
Although spontaneous formation of polytene chromosomes has been reported in the current study, further studies on the occurrence of endoreduplication in pollen grains of other angiosperms are necessary to strengthen these findings and to explain their evolutionary and ecological significance.
Conflict of interest
The author declares no conflict of interest.
Notes on contributor
Sevil Tütüncü Konyar is a botanist in Turkey. Her areas of expertise are palynology, plant cell, ultrastructure, pollen ontogeny, plant embryology and plant morphology.
Acknowledgements
The author is deeply grateful to Dr Serpil Tütüncü and Ercüment Konyar for their help during the collection and fixation of the material. Transmission electron microscopy studies and sectioning of the semi-thin sections have been carried out in the Plant, Drug and Scientific Researches Centre of Anadolu University (AUBIBAM). The author would also like to thank the manager of the Scientific Researches Centre, Professor Lütfi Genç, and the assistant manager, Associate Professor Deniz Hür, for their kind help during the execution of the study.
References
- Ashburner, M. 1970. “Function and structure of polytene chromosomes during insect development.” Advances in Insect Physiology 1: 1–95.
- Barow, M. 2006. “Endopolyploidy in seed plants.” Bioessays 28 (3): 271–81.
- Brodsky, V. Y. A., and I. Uryvaeva. 1985. Genome Multiplication in Growth and Development Biology of Polyploid and Polytene Cells. Cambridge, UK: Cambridge University Press.
- Brady, T. 1973. “Feulgen cytophotometric determination of the DNA content of the embryo proper and suspensor cells of Phaseolus coccineus.” Cell Differentiation 2: 65–67.
- Brady, T., and M. E. Clutter. 1974. “Structure and replication of Phaseolus polytene chromosomes.” Chromosoma 45: 63–79.
- Carvalheira, G. M. G., and M. Guerra. 1994. “The polytene chromosomes of anther tapetum of some Phaseolus species.” Cytologia 59: 211–217.
- Carvalheira, G. M. G. 2000. “Plant polytene chromosomes.” Genetics and Molecular Biology 3: 1043–1050.
- Ceccarelli, M., E. Sanantonio, F. Marmottini, G. N. Amzallag, and P. G. Cionini. 2006. “Chromosome endoreduplication as a factor of salt adaptation in Sorghum bicolor.” Protoplasma 227: 113–118.
- Chevalier, C., M. Nafati, E. Mathieu-Rivet, M. Bourdon, N. Frangne, C. Cheniclet, J. P. Renaudin, F. Gévaudant, and M. Hernould. 2011. “Elucidating the functional role of endoreduplication in tomato fruit development.” Annals of Botany 107: 1159–1169.
- Chojecki, A. J. S., M. W. Bayliss, and M. D. Gale. 1986. “Cell production and DNA accumulation in the wheat endosperm and their association with grain weight.” Annals of Botany 58: 809–827.
- Cionini, P. G., A. Cavallini, R. Cosi, and M. Fogli. 1982. “Comparison of homologous polytene chromosomes in Phaseolus coccineus embryo suspensor cells: morphological, autoradiographic and cytophotometric analyses.” Chromosoma 86: 386–396.
- Craig, E. L., W. J. Frojola, and M. H. Greider. 1962. “An embedding technique for electron microscopy using epon 812.” The Journal of Cell Biology 12 (1): 190–194.
- Comai, L. 2005. “The advantages and disadvantages of being polyploid.” Nature Reviews Genetics 6: 836–846.
- Cookson, S. J., A. Radziejwoski, and C. Granier. 2006. “Cell and leaf size plasticity in Arabidopsis: What is the role of endoreduplication?” Plant, Cell and Environment 29: 1273–1283.
- D’Amato, F. 1984. “Role of polyploid in reproductive organs and tissues.” In Embryology of Angiosperms, edited by B. M. Johri, 519–566. Berlin: Springer-Verlag.
- D’Amato, F. 1989. “Polyploidy in cell differentiation.” Caryologia 42: 183–211.
- De Castro, O., S. Brullo, P. Colombo, S. Jury, P. De Luca, and A. Di Maio. 2012. “Phylogenetic and biogeographical inferences for Pancratium (Amaryllidaceae), with an emphasis on the Mediterranean species based on plastid sequence data.” Botanical Journal of the Linnean Society 170: 12–28.
- Donovan, G. R. 1979. “Relationship between grain nitrogen, non-protein nitrogen and nucleic acids during wheat grain development.” Australian Journal of Plant Physiology 6: 449–457.
- Durbarry, A., I. Vizir, and D. Twell. 2005. “Male germ line development in Arabidopsis: duo pollen mutants reveal gametophytic regulators of generative cell cycle progression.” Plant Physiology 137: 297–307.
- Ekim, T., M. Koyuncu, M. Vural, H. Duman, Z. Aytaç, and N. Adıgüzel. 2000. Red Data Book of Turkish Plants (Pteridophyta and Spermatophyta). Barışcan Ofset: Turkish Association for the Conservation of Nature-Van Centennial University, Ankara.
- Evers, A. D. 1970. “Development of the endosperm of wheat.” Annals of Botany 34: 547–555.
- Freed, H. J., and W. F. Grant. 1976. “Polytene chromosome in the suspensor cells of Lotus (Fabaceae).” Caryologia 29: 387–390.
- Gostev, A., and S. Asker. 1978. “Polytene chromosomes in glandular hairs of Salvia horminum.” Hereditas 88: 133–135.
- Guerra, M., and G. M. G. Carvalheira. 1994. “Occurrence of polytene chromosomes in the anther tapetum of Vigna unguiculata L. (Walp.).” Journal of Heredity 85: 43–46.
- Hasischka-Jenschke, G. 1957. “Die Entwicklung der Samenanlage von Allium ursinum mit besonderer Berücksichtigung der endopolpyploiden Kerne in Synergiden und Antipoden.” [Ovule development in Allium ursinum with special emphasis on endoploid nuclei in synergids and antipodes.] Österreichische Botanische Zeitschrift 104:1–24
- Johri, B. M., K. B. Ambegaokar, and P. S. Srivastava. 2013. Comparative Embryology of Angiosperms. vol. 1. Berlin: Springer-Verlag.
- Khanna, P. 2010. Essentials of Genetics. New Delhi, India: I. K. International Pvt.
- Kowles, R. V., and R. L. Phillips. 1985. “DNA amplification patterns in maize endosperm nuclei during kernel development.” Proceedings of the National Academy of Sciences USA 82: 7010–7014.
- Kumar, G., and S. Verma. 2011. “Presence of Polytene nuclei with chromocenters un associated with chromatin bundles in Vigna unguiculata.” Chromosome Botany 6: 17–19.
- Mabberley, D. J. 2008. Mabberley’s Plant-Book. 3rd ed. Cambridge: Cambridge University Press.
- Mill, R. R. 1984. “Pancratium L.” In Flora of Turkey and the East Aegean Islands, edited by P.H Davis, vol. 8, 380–381. Edinburgh: Edinburgh University Press.
- Nadirashvili, N., G. Gvaladze, and M. Akhalkatsi. 2006. “Structure and function of the hypertrophic synergid in some species of genus Allium L.” Proceedings of the Georgian Academy of Sciences, Biological Series B 4 (2): 53–60.
- Nagl, W. 1969. “Banded polytene chromosomes in the legume Phaseolus vulgaris.” Nature 221: 70–71.
- Nagl, W. 1970. “Inhibition of polytene chromosome formation in Phaseolus by polyploid mitosis.” Cytologia 35: 252–258.
- Nagl, W. 1974. “The Phaseolus suspensor and its polytene chromosomes.” Zeitschrift für Pflanzenphysiologie 73: 1–44.
- Nagl, W. 1976. “The polytenic antipodal cells Scilla bifolia: DNA replication pattern and possibility of nucleolar DNA amplification.” Cytobiologie 14: 165–170.
- Nagl, W. 1978. Endopolyploidy and Polytene in Differentiation and Evolution. Amsterdam: North-Holland Publishing.
- Nagl, W. 1981. “Polytene chromosomes of plants.” International Review of Cytology 73: 21–53.
- Nagl, W. 1987. “Genetics: I. Replication.” Progress in Botany 49: 181–188.
- O’Brien, T. P., N. Feder, and M. E. McCully. 1964. “Polychromatic staining of plant cell walls by toluidine blue.” Protoplasma 59: 368–373.
- Petrova, T. F., O. B. Solovyanova, and Y. U. S. Chentsov. 1985. “Ultrastructure of giant chromosomes in barley antipodes.” Cytologia 5: 499–503.
- Pearson, M. J. 1974. “The abdominal epidermis of Calliphora erythrocephala (Diptera): I. Polyteny and growth of the larval cells.” Journal of Cell Science 16: 113–131.
- Rajya Lakshmi, T. V., C. H. Nimmi, K. G. Raja Rao, P. S. R. L. Narasinga Rao, and N. R. Isola. 2001. “Banded polytene chromosomes in developing endosperm of pearl millet.” Journal of Heredity 92 (4): 360–361.
- Ramachandran, C., and V. Raghavan. 1989. “Changes in nuclear DNA content of endosperm cells during grain development in rice (Oriza sativa).” Annals of Botany 64: 459–468.
- Ribbert, D. 1979. “Chromomeres and puffing in experimentally induced polytene chromosomes of Calliphora erythrocephala.” Chromosoma 74: 269–298.
- Scholes, D. R., and K. N. Paige. 2015. “Plasticity in ploidy: A generalized response to stress.” Trends in Plant Science 20 (3): 165–175.
- Sunderland, N., G. B. Collins, and J. M. Dunwell. 1974. “The role of nuclear fusion in pollen embryogenesis of Datura innoxia Mill.” Planta 117: 227–241.
- Şenel, G., M. Özkan, and N. Kandemir. 2002. “A karyological investigation on some rare and endangered species of Amaryllidaceae in Turkey.” Pakistan Journal of Botany 34 (3): 229–235.
- Zhimulev, I. F., and D. E. Koryakov. 2009. “Polytene Chromosomes.” In Encyclopedia of Life Sciences (ELS), 1–10. Chichester: John Wiley and Sons.
- Zybina, T., and E. Zybina. 2011. Cell Cycle Modification in Trophoblast Cell Populations in the Course of Placenta Formation, DNA Replication and Related Cellular Processes. InTech, Available from: http://www.intechopen.com/books. (Accessed 17 March 2015).
