Abstract
A new species Arthrocnemum franzii Sukhor. is described from the Republic of Cape Verde (Sal, Maio and Boa Vista islands). The species is recognized as distinct from Arthrocnemum macrostachyum (Moric.) K.Koch based on differences in the perianth shape, length of the anthers and style, and seed-coat ornamentation. No seed heteromorphism is observed within individuals of either species, despite differences in the size of the central and lateral flowers within each cyme (heteroanthocarpy). The North American Arthrocnemum subterminale (Parish) Standl. (syn. Salicornia subterminalis Parish) is morphologically distant from Eurasian Arthrocnemum or Salicornia/Sarcocornia group and should be excluded from these genera. The genus Arthrocnemum now comprises only two species (A. macrostachyum and A. franzii), distributed in the Mediterranean area, Macaronesia, West Tropical Africa and the Saharo-Arabian region. A generic description is here elaborated, clearly delimiting Arthrocnemum from morphologically similar species of Sarcocornia. A list of current species previously considered as Arthrocnemum is provided. It is argued that the taxonomic status of Salicornia mucronata Lag. (1817), mentioned in some references as a synonym of Salicornia macrostachya Moric. (1820) [≡Arthrocnemum macrostachyum (Moric.) K.Koch], is indeed a new synonym of Anabasis articulata (Forssk.) Moq. (subf. Salsoloideae). Both names merged with Arthrocnemum macrostachyum – Salicornia virginica Forssk. and Arthrocnemum glaucum (Delile) Ung.-Sternb. var. fasciculatum Sennen were lectotypified. The typification of the genus Arthrocnemum has so far been lacking and requires a special proposal with a conserved type.
Introduction
The genus Arthrocnemum Moq. belongs to the taxonomically and diagnostically difficult subfamily Salicornioideae, which differs from many other Chenopodiaceae-Amaranthaceae groups by the presence of fleshy, opposite or alternate leaves usually reduced to scales and often looking like bracts, the absence of bracteoles, and the flowers arranged in cymes consisting of three (one central and two lateral) flowers of whitish or brownish colour. Arthrocnemum was described by Moquin-Tandon (Citation1840) who included several shrubby species of similar habit and reproductive characters (e.g. crustaceous seed coat and mealy perisperm in the seed). Both carpological traits indicated in the protologue are indeed variable in Arthrocnemum. In its earlier circumscription (e.g. Ball Citation1964; Aellen, Cullen and Coode 1967) Arthrocnemum also includes shrubby species of Salicornia L. However, the latter genus is characterized as distinct from Arthrocnemum by the limited amount of nutritive tissue present in the seed and the hair-like or papillate outgrowths of the seed testa cells, which do not contain tannin-like outgrowths (so-called ‘stalactites’) on the outer wall (Bunge Citation1856; Brenan Citation1954b; Castroviejo and Coello Citation1980; Shepherd, Macfarlane and Colmer Citation2005a; Sukhorukov Citation2014).
In the past Arthrocnemum was considered to be a genus of several (three to ten) species distributed in Africa, America and Australia (Moquin-Tandon Citation1840; Koch Citation1853; Ungern-Sternberg Citation1866, Citation1876; Volkens Citation1893; Standley Citation1914; Brenan Citation1954a; Scott Citation1977; Meikle Citation1985; Kühn Citation1993). The nomenclatural confusion in the naming of the ‘Arthrocnemum – shrubby Salicornia group’ remained in many past treatments (Moss Citation1954; Ball Citation1964; Toelken Citation1967), until Scott (Citation1977) typified the genus Arthrocnemum with Arthrocnemum fruticosum (L.) Moq. var. macrostachyum (Moric.) Moq., the variety mentioned by Moquin-Tandon (Citation1840), which most closely matched the diagnosis of the genus. It should be pointed out that the lectotypification undertaken by Scott (Citation1977) is incorrect as it is based on a name of non-specific rank (cf. Hedge Citation1997; ICN Citation2012 [Art. 10.1]), and none of the species recognized by Moquin-Tandon (Citation1840) is appropriate for the typification. This nomenclatural problem needs to be addressed separately, together with the proposed conservation of the name Arthrocnemum with the type Arthrocnemum macrostachyum (Moric.) K. Koch (≡ A. fruticosum (L.) Moq. var. macrostachyum Moq.) as type.
All shrubby taxa from Southern Africa formerly assigned to Arthrocnemum (Hiern, Rendle and Welwitsch Citation1896; Moss Citation1954; Toelken Citation1967; Lebrun and Stork Citation1991) or even Arthrocnemum macrostachyum reported from Angola (Baker and Clarke Citation1913; Lebrun and Stork Citation2003) belong in fact to various Sarcocornia species (O’Callaghan and Oliver Citation1992; Kadereit, Mucina and Freitag Citation2006), and thus Arthrocnemum is not represented in Southern Africa (Steffen, Mucina and Kadereit Citation2009, Citation2010). The same applies to the European (A. fruticosum, Arthrocnemum perenne) and South American (A. fruticosum auct.) species previously included in Arthrocnemum and now included in Sarcocornia (Davy et al. Citation2006; Alonso and Crespo Citation2008). Arthrocnemum fruticosum (L.) Moq. and Arthrocnemum ambiguum (Michx.) Moq. are now considered as Sarcocornia fruticosa (L.) A.J. Scott and Sarcocornia ambigua (Michx.) M.A. Alonso & M.B. Crespo respectively (Scott Citation1977; Alonso and Crespo Citation2008); Arthrocnemum belangerianum Moq. is now Halostachys belangeriana (Moq.) Botsch. (Botschantzev Citation1954; Sukhorukov Citation2014), and both Arthrocnemum arbuscula (R.Br.) Moq. and Arthrocnemum indicum (Willd.) Moq. are currently included in Tecticornia Hook.f. (Shepherd and Wilson Citation2007).
Among these taxa, Arthrocnemum macrostachyum and Sarcocornia fruticosa are often found growing together in the Mediterranean area in the same ecological conditions associated with marshes and other saline supralittoral or inland habitats (Jalas and Suominen Citation1980; Kühn Citation1993; Guilló et al. Citation2014; Sukhorukov, pers. obs. in Cyprus and Israel), and they are often confused with each other. Indeed, many morphological or even anatomical characters of both articulated Arthrocnemum and Salicornia including paraphyletic Sarcocornia (Shepherd, Macfarlane and Waycott Citation2005b; Steffen et al. Citation2015) are overlapping (Zare and Keshavarzi Citation2007). Despite many morphological homologies (shrubby life history; glabrous stem; opposite scale-like leaves and bracts; cymes consisting of three flowers; rupture of the lower part of both perianth and pericarp making the ripe seed free; vertical embryo position) and similar (saline) habitats, there are several important traits unambiguously distinguishing Arthrocnemum from the Salicornia/Sarcocornia group (Table ; see also De Fraine Citation1913; Ferguson Citation1964; Sukhorukov Citation2014). In addition to the data presented in Table , it should be pointed out that the colour of many Sarcocornia varies from green to reddish, whereas both A. macrostachyum and Arthrocnemum franzii are always grey or (in some cases) yellowish (Sennen Citation1936; Sukhorukov, pers. obs. in Cape Verde). Additionally, some articulated Salsoloideae (e.g. Anabasis) habitually resemble Arthrocnemum or the Salicornia/Sarcocornia group and are often confused in herbaria (see also taxonomic comments below under Salicornia mucronata Lag.), but the representatives of both subfamilies are very dissimilar in stem anatomy (epidermal layers: Dangeard Citation1887; De Fraine Citation1913; O’Callaghan Citation1992; Milič et al. Citation2011; Grigore, Ivanescu and Toma 2014) or reproductive characters, especially fruit anatomy (Sukhorukov Citation2008, with further references herein; Sukhorukov et al. Citation2015).
Table 1. Differences between Arthrocnemum and Salicornia/Sarcocornia.
Currently, a single North American species – Arthrocnemum subterminale (Parish) Standl. – is included in Arthrocnemum (Standley Citation1914; Ball Citation2003). This species appears to be unrelated to A. macrostachyum or A. franzii on account of differences in the perianth, pericarp and seed characters, and deserves further investigation concerning its taxonomic status (compare Parish Citation1898; Wilder, Felger and Romero-Morales Citation2008, both references as Salicornia; or Standley Citation1914; Ball Citation2003 as Arthrocnemum). We state here for the first time that this species possesses a unique perianth that splits longitudinally into two parts in the fruiting stage (essentially making the fruit free), as well as having a thinner pericarp and brown (not black as in Eurasian Arthrocnemum) seeds with no papilla-like outgrowths. Steffen et al. (Citation2015) suggest that A. subterminale is not related to Arthrocnemum s.str.
Another species, Arthrocnemum indicum, which is widely distributed in the coastal zones of the Indian Ocean (East and Southern Africa, Sub-Indian continent and Australia), has been transferred to Halosarcia Wilson (Wilson Citation1980) and then to Tecticornia Hook.f., a genus with the highest diversity in Australia (Shepherd, Waycott and Calladine Citation2004; Shepherd Citation2007; Shepherd and Wilson Citation2007). The differences between Arthrocnemum and Tecticornia (especially Tecticornia indica previously considered to be Arthrocnemum indicum) were clarified by Wilson (Citation1980) based on the anther number (two anthers versus one abaxial anther, respectively). Other distinguished characters of T. indica are the rooting stem, flowers connate to each other (but details lacking where both flower types are located in the inflorescence: Jafri and Rateeb Citation1978, sub Arthrocnemum indicum), and pale brown seeds (Friis and Gilbert Citation1993, as Halosarcia indica).
Both molecular (e.g. Shepherd, Waycott and Calladine, Citation2004; Kadereit, Mucina and Freitag, Citation2006; Kadereit and Yaprak Citation2008) and carpological (Sukhorukov Citation2014) results show that Arthrocnemum is closely related to the monotypic genus Microcnemum found in the Mediterranean region. The genera are nested within one clade (Shepherd, Macfarlane and Waycott, Citation2005b; Kadereit, Mucina and Freitag, Citation2006; Kadereit and Yaprak Citation2008), which is clearly distant from both Salicornia and Sarcocornia, which together form a separate lineage. Carpologically both Arthrocnemum and Microcnemum possess black seeds with a thick, crustaceous coat often forming stout (papilla-like) outgrowths from the testa cells, and with “stalactites” on the outer cell walls of the testa (Sukhorukov Citation2014). Both genera are well-distinguished by the life history (shrubby habit in Arthrocnemum and annual habit in Microcnemum).
The genus Arthrocnemum has recently been considered to comprise only A. macrostachyum (Hedge Citation1997; Yaprak Citation2008), which is a widely distributed species in the Mediterranean basin with extensions into the Macaronesian, Saharo-Arabian and Irano-Turanian floristic regions, or to also include the North American A. subterminale (Standley Citation1914; Ball Citation2003). We recognize that the reproductive organs in Arthrocnemum, especially the perianth and pericarp, have not been sufficiently studied to date. In the present paper we devote particular attention to their taxonomic significance in the genus, and draw comparisons with similar-looking taxa. Besides, A. macrostachyum is morphologically heterogeneous and is re-circumscribed to exclude a new species from Macaronesia, which is recognized herein as A. franzii Sukhor.
Material and methods
The Arthrocnemum material was collected by A. Sukhorukov in Cyprus (November 2006) and in Cape Verde (August–September 2015, January 2016). Additionally, the first author has examined the Arthrocnemum material in the herbaria B, BM, BR, E, HUJ, K, LE, MHA and MW [herbarium abbreviations according to Thiers (Citation2008+)], and has used some specimens for comparative study (see Appendix 1). The perianth sections were obtained using a microtome (embedding the perianth and pericarp in Technovit), with no staining of the sections. The images of the seed ultrasculpture were made using a scanning electron microscope (SEM) JSM–6380 (JEOL Ltd., Japan) at 15 kV. The seeds were cut by hand or with a microtome (no dye is needed for the seeds because all the seed-coat cells are impregnated with tannins). All the images were taken with the camera Carl Zeiss AxioCam MRc using light microscope Carl Zeiss Axioplan 2.
Results
As a result of our studies, we consider that the populations in Cape Verde are distinguished from Mediterranean A. macrostachyum by several important reproductive characters and deserve recognition at species rank. This raised the need for a new generic delimitation of the genus Arthrocnemum, which is provided in the taxonomic treatment below. Additionally, a checklist of all the names assigned to Arthrocnemum is given.
Taxonomic treatment
Arthrocnemum Moq., Chenop. Monogr. Enum.: 111 (1840).
Type of the genus: not yet typified (the genus should be typified with a conserved name).
The genus Arthrocnemum can be morphologically characterized by the following distinct characters:
Shrubs to 1.5 m tall, very branched from the base often forming mats; annual shoots glaucous (sometimes yellowish), glabrous (or one-layered epidermis can be represented by mamillate cells). Phyllotaxis decussate; leaves reduced to opposite, basally concrescent and cuspidate scales up to 5 mm long, with fissures between the scales within one node. Inflorescence on lateral branches terminal, not branching or with short paraclades; each cyme of three flowers subtended by a bract (each node contains two opposite bracts and hence six flowers). Flowers bisexual (sometimes the stamens in the lateral flowers are missing and so they may be pistillate only), protandric. Perianth always concrescent to the apex (sometimes with small terminal lobes), directed upwards, protruding by up to one-third to one-half the length of the bracts and consisting of parenchymatous, thin-walled cells arranged in several layers, with inclusion of scattered lignified cells as an inner layer, but never completely indurated, and with no tracheoidioblasts; perianth of the central flower four-angled (probably consisting of four segments), and that of the two lateral (peripheral) flowers three-angled (heteroanthocarpy). Ovary conical, fruit wall (pericarp) of parenchymatous cells, thick and few-layered in upper part and thinning in lower portion. Stamen 1–2, anthers protruding from the perianth, 0.8–1.3 mm. Style present, with two stigmas. Seed black (reddish when unripe), testa crustaceous, mostly with papilla-like (conic) outgrowths located along one (embryo-bearing) side, 20–25 μm thick (in flattened cells) and up to 55 μm thick in conic cells, their outer wall bearing five to ten stalactites, cell content easily visible. Perisperm present. Embryo curved (comma-shaped), vertical, radicle in abaxial position, cotyledons located adaxially (close to the axis).
Morphological notes
We cannot confirm the statement that the lateral flowers in Arthrocnemum are staminate only (Kühn Citation1993) and so do not produce fruit. In fact all (central and both lateral) flowers in the cyme of A. macrostachyum and A. franzii sp. nov. (description of a new species is given below) possess a well-developed ovary, although they often remain sterile (for example, in the populations of A. franzii seen in Cape Verde in 2015). The causes of the sterility are still not known. The presence of an indurated pericarp in Arthrocnemum, mentioned by Ungern-Sternberg (Citation1866), Scott (Citation1977), Jafri and Rateeb (Citation1978) or Friis and Gilbert (Citation1993) has also been shown to be unfounded.
1. Arthrocnemum macrostachyum (Moric) KKoch, Hort Dendrol: 96 (1853)
The same combinations of Moris and Delporte (Citation1854) and Bunge in Ungern-Sternberg (Citation1866) are superfluous.
Bas.: Salicornia macrostachya Moric., Fl. Venet. 1: 2 (1820).
Holotype: Des environs de Venise [surroundings of Venice], Malamocco, herb. Moricand (G – photo!).
≡ Salicornia virginica Forskål, Fl. Aegypt.-Arab.: 2 (1775) nom. illegit. non L. (1753);
Described from Egypt. Lectotype (Sukhorukov, designated here): “Circa Alexandriam [leg.] Forskål 174” (C-10002990 – photo!).
≡ S. glauca Delile, Fl. Egypt: 69 (1813) nom. illegit. non Stokes (1812).
Described from Egypt without precise location (holo – LINN-HS20-13, photo!).
≡ Arthrocnemum fruticosum (L.) Moq. γ [var.] macrostachyum Moq., Chenop. Monogr. Enum.: 111 (1840).
≡ A. glaucum (Delile) Ung.-Sternb., Atti Congr. Bot. Firenze: 283 (1876);
≡ A. glaucum (Delile) Ung.-Sternb. var. fasciculatum Sennen, Diagn. Nouv. Exs.: 204 (1936).
Lectotype (Sukhorukov, designated here): Maroc, Melilla, a la Bocana, 24 November 1932, leg. Sennen & Mauricio 8917 (BM; iso–BC-141886, MPU-009401, MPU-009401 – photo!). The plants of yellowish colour are collected in the late fruiting stage with condensed inflorescence (Sennen Citation1936). The seeds observed from collection in BM possess a small amount of the conic (papilla-like) cells.
≡ A. indicum (Willd.) Moq. subsp. glaucum (Delile) Maire & Weiller in Maire, Fl. Afr. Nord. 8: 99 (1962).
Taxonomic note
We argue that the still unresolved and forgotten Salicornia mucronata Lag. described from Spain (Lagasca Citation1817), cited in some references as a synonym of Arthrocnemum macrostachyum (Ungern-Sternberg Citation1876; Rouy Citation1910; Zohary Citation1966), is indeed a new synonym of Anabasis articulata (Forsk.) Moq. The holotype specimen of Salicornia mucronata kept at MA (photo!) is represented by a young branch with mucronate scale-like leaves (like in Arthrocnemum), but possesses the tufts of the simple hairs in the leaf axils (report of Charo Noya Santos) that is one of the most remarkable characters in the tribe Salsoleae, subf. Salsoloideae (Sukhorukov Citation2014).
2. Arthrocnemum franzii Sukhor. sp. nov. (Figure )
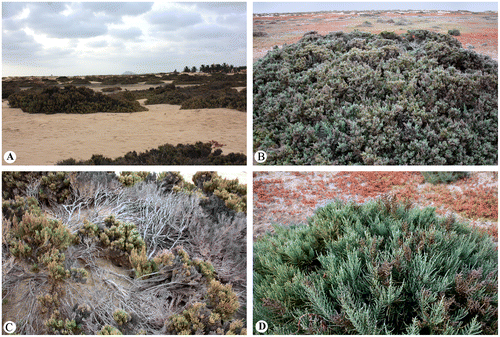
Figure 1. General view of Arthrocnemum franzii in Sal Island (August–September 2015). (A) Dominant in the sandy depressions (often together with Sesuvium sp., Suaeda vermiculata and Zygophyllum waterlotii). (B) Closer look at an individual. (C) Branching pattern of the new species. (D) Part of the plant with fruiting inflorescence. Photographer: A. Sukhorukov.
Shrub up to 1 m tall forming mats up to 3 m across, often with twisted perennial shoots; annual branches glaucous (sometimes yellowish) and glabrous. Leaves up to 5 mm long, cup-like, acuminate. Inflorescence cylindrical, to 10 cm, consisting maximum of 50 nodes, branched in lower parts or not, with short paraclades (if present). Bracts similar to leaves, not fused to the perianth. Flowers 3 in a cyme, free. Perianth of the central flowers four-angled, trapezoid, 1.5–1.7 mm long; perianth of lateral flowers three-angled, forming a conus, 1.3–1.5 mm long; one-third to one-half of all flowers protruding above the subtending bract. Stamen 1–2, anther 0.8–1.0 mm long. Ovary 1.3–1.6 mm long, gradually tapering to the style 1–1.5 mm long with two stigmas ~ 1 mm long. Fruit embedded in the perianth, with hyaline pericarp. Seeds developing in all central and lateral flowers, 1.0–1.3 mm long, 0.7–0.8 mm wide, 0.5–0.6 mm thick, black, generally with easily visible papilla-like outgrowths located along the embryo-bearing seed margin; testa cells (20)25 to 55 μm (the thickness depending on whether the cells have such outgrowths), their outer cell walls with five to ten stalactites. Perisperm copious. Embryo comma-shaped.
Holotype (Figure ): Republic of Cabo Verde, Sal Island, 2 km west from Santa Maria town, 16°59'02.46" N, 22°92'42.72" W, sandy depressions near the sea, 30 August 2015, Alexander P. Sukhorukov 56 (MW-0198220 ! iso – BR, L, LE).
Additional specimens seen: Republic of Cabo Verde: Sal Island, [Rifes da] Parda, June 1934, A. Chevalier s.n. (K); Sal Island, Santa Maria, 19 October 1934, M. Dinklage 3194 (BM); Maio Island, Terra Salgadas Salinas N of Morrinho [c. 15°16'40" N, 23°12'30" W], seasonally flooded plain on the landward side of the coastal dune belt, 4 January 1994, N. Kilian & T. Leyens NK3028 (B); Boa Vista Island, Sal Rei, 16°18'40.95" N, 22°91'66.38" W, salty plain on the landward side of the coastal dune belt, 10 January 2016, A.P. Sukhorukov & A. Konstantinova 683 (G, M, MW, W); Boa Vista, 8 km south of Sal Rei, 16°11'71.42" N, 22°90'29.04" W, sandy depressions near the sea, 10 January 2016, A.P. Sukhorukov & A. Konstantinova 693 (MW); Boa Vista, Santa Monica beach, 15°98'41.77" N, 22°84'24.79" W, seasonally flooded plain on the landward side of the coastal dune belt, 10 January 2016, A.P. Sukhorukov & A. Konstantinova 697 (MW).
On the Sal and Boa Vista Islands, this species was previously called Arthrocnemum fruticosum (Schmidt Citation1852) as well as A. macrostachyum (Martins Citation2002).
Distribution
The new species is distributed in Tropical West Africa – Cape Verde (Boa Vista, Maio, Sal as the most arid islands in the archipelago) (Figure ). It is likely that the ranges of A. macrostachyum (Mediterranean area with extensions to the Saharo-Arabian floristic province) and A. franzii do not overlap.
Ecology and plant communities
Seasonally flooded, saline plain on the landward side of the coastal dune belt.
In Sal and Boa Vista Islands, the species is a dominant of the depressions in natural sandy landscapes near sea level, and grows together with Suaeda vermiculata Forssk. ex J.F. Gmel. (Amaranthaceae–Chenopodiaceae), Sesuvium sp. (Aizoaceae) incorrectly identified in the herbaria BM, K as S. portulacastrum L., and Tetraena gaetula (Emb. & Maire) Beier & Thulin subsp. waterlotii (Maire) Beier & Thulin (≡ Zygophyllum waterlotii Maire) (Zygophyllaceae). In contrast to all the species mentioned, Arthrocnemum franzii do not appears as a constituent of disturbed plant communities.
Flowering and fruiting
Flowering: January–May; fruiting: May–July.
IUCN Red List Category
Although appropriate data on abundance and/or distribution of the taxon are lacking for Maio Island, we recorded A. franzii as common on sandy inland plains on Sal and Boa Vista. However, the construction of new buildings elsewhere in Cape Verde (especially hotels on Sal as one of the most visited islands in the archipelago) drastically damages the natural landscapes (Romeiras et al. Citation2016; A. Sukhorukov, pers. obs.), and additionally the plants investigated produce few fruits (not more than 5% of flowers are fertile). We accordingly recommend inclusion of the new species in one of the Red List categories (IUCN Citation2014), but the exact categorization must be decided only after further detailed studies in other islands of Cape Verde. Till now, Arthrocnemum is not included in the list of threatened species in Cape Verde archipelago (Romeiras et al. Citation2016).
Etymology
The species is named after Baron Franz Ungern-Sternberg (1808–1885), botanist and physician, and expert on the Salicornioideae, Chenopodiaceae (see also Quattrocchi Citation2000).
Taxonomic notes
The new species is distinguished from A. macrostachyum by several reproductive characteristics, and these can be used for delimiting the species and refining the genus characterization. The species are compared with respect to each character.
Perianth
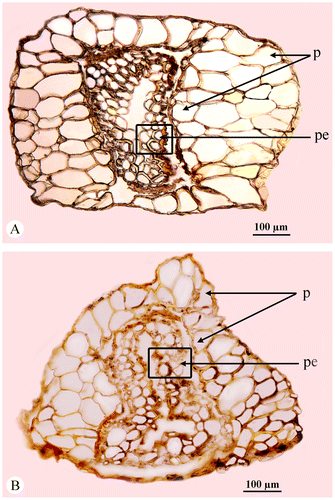
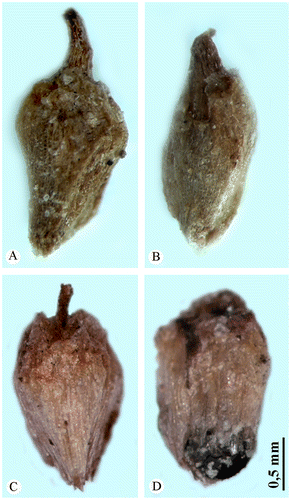
The perianth of the central and lateral flowers of both species consists of parenchymatous, often spongy and never indurated cells lacking tracheoidioblasts. Both A. macrostachyum and A. franzii are characterized by differences in perianth shape of three-flowered cyme (trapezoid perianth in the central flower and conus-like perianth in both lateral flowers), termed heteroanthocarpy (Sukhorukov Citation2010) (Figure ). However, A. macrostachyum and A. franzii differ from each other in the outlines of the perianth shape of the central flowers (obconic in A. macrostachyum with small ear-like appendages (terminal lobes), and trapezoid perianth with no appendages in the latter species).
Figure 4. Flowers (front view). (A) Central flower in the cyme of Arthrocnemum franzii. (B) Lateral flower in the cyme of A. franzii. (C) Central flower in the cyme of Arthrocnemum macrostachyum. (D) Lateral flower in the cyme of A. macrostachyum. Origin of the material: (A, B) Alexander P. Sukhorukov 56 (holotype) and (C, D) H. Freitag, Egypt, 1987 (LE).
Anthers
Field-based collections by the first author confirm that the anthers of A. franzii are shorter (0.8–1 mm long) than those observed in A. macrostachyum s.str. (1–1.3 mm) growing in the Mediterranean area, which is in agreement with previous data for A. macrostachyum (Maire Citation1962; Castroviejo Citation1990).
Style
Arthrocnemum franzii is characterized by the longer (1.0–1.5 mm), easily visible, thick style (Figure , A) that splits into 2(3) stigmas of the same length. In contrast, A. macrostachyum is distinguished by the clearly shorter (0.4–0.7 mm) style, that hardly protrudes beyond the perianth, and the 2(3) stigmas being 1.0–1.5 mm long (Figure , B); see Castroviejo Citation1990). At the final fruiting stage, the stigmas and upper part of the style break off, with the lower portion persisting. This protrusion is approximately 0.5–0.7 mm long in A. franzii, which is twice the length of that observed in A. macrostachyum (0.25–0.4 mm).
Figure 6. Fruiting inflorescence in Arthrocnemum franzii (A) and Arthrocnemum macrostachyum (B). Abbreviations: sl–style (A. franzii), stigmas fallen off; st – stigmas (A. macrostachyum); style not protruding. Origin of the material: A. franzii from the holotype (Cape Verde), A. macrostachyum: Israel, Dead Sea, 1902, J.E. Dinsmore 9102 (HUJ).

Seeds
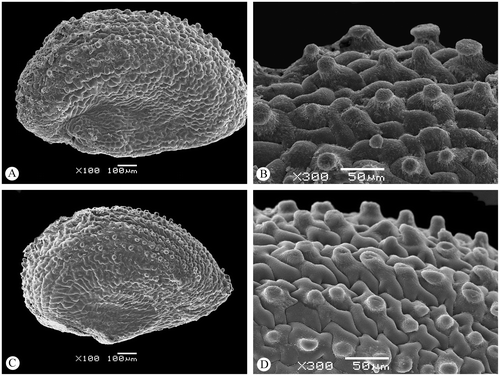
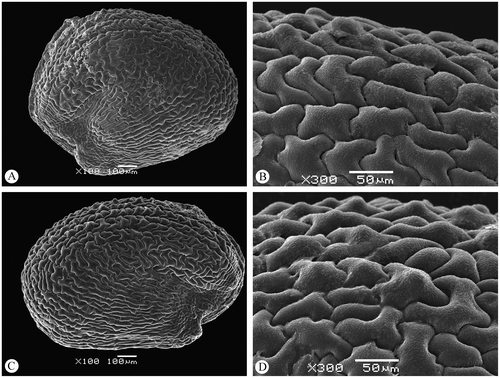
The seeds of both A. franzii and A. macrostachyum are morphologically and anatomically monomorphic. The length of the seeds in A. franzii is 1.1–1.3 mm, and the length/width ratio is 2 : 1 (the seeds are clearly elongated; for comparison see also Guilló, Alonso and Juan 2013). The seeds of A. macrostachyum are 1.0–1.1 mm long and 0.7–0.9 mm wide (length/width ratio 1.2–1.5 : 1). Moreover, the number of papilla-like outgrowths of the testa cells (mostly arranged along the embryo-containing part of the seed) in A. franzii is usually much greater than in A. macrostachyum (Figures ).
Conclusion
To conclude the discussion of the taxonomic diversity of Arthrocnemum, we reiterate that the genus as defined by us comprises only two taxa – A. macrostachyum (Moric.) K.Koch, mostly widespread in the Mediterranean area and the northern Sahara (White Citation1983; Greuter, Burdet and Long 1984), and a new species A. franzii Sukhor. in West Africa. However, the populations in the coastal regions of the Arabian Sea with an unusual habit, especially on Socotra archipelago (Yemen, see Brown and Mies Citation2012), as well as in Sudan, Somalia and Ethiopia deserve a closer look, since the existing material is inadequate, and did not allow us to assess its taxonomic status or assign it to A. macrostachyum. We surmise that A. subterminale should be transferred to a new genus (based on the reproductive characters), and this suggestion will be supported by the preliminary molecular data (Steffen et al. Citation2015).
Checklist of Arthrocnemum names
We set out the names previously known as Arthrocnemum and which have recently been assigned to other genera, especially to Sarcocornia. We mostly cite here the most recent references due to disambiguation of some names – e.g. Arthrocnemum africanum in Scott (Citation1977) and Steffen, Mucina and Kadereit (2010). The accepted names are highlighted in bold although, in the light of the paraphyly of Sarcocornia (Shepherd, Macfarlane and Awaycott Citation2005b; Kadereit, Mucina and Freitag, Citation2006; Steffen et al. Citation2015), all species now assigned to this genus should apparently be recognized as belonging to an extended Salicornia.
Arthrocnemum affine Moss ≡ Sarcocornia natalensis (Bunge ex Ung.-Sternb.) A.J. Scott subsp. affinis (Moss) S. Steffen, Mucina & G. Kadereit (Steffen et al. Citation2010);
A. africanum Moss ≡ Sarcocornia natalensis (Bunge ex Ung.-Sternb.) A.J. Scott (Steffen et al. Citation2010);
A. ambiguum (Michx.) Moq. ≡ Sarcocornia ambigua (Michx.) M.A. Alonso & M.B.Crespo (Alonso and Crespo Citation2008);
A. arbuscula (R.Br.) Moq. ≡ Tecticornia arbuscula (R.Br.) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. australasicum (Moq.) Moss ≡ Tecticornia australasica (Moq.) (Paul G. Wilson) K.A.Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. belangerianum Moq. ≡ Halostachys belangeriana (Moq.) Botsch. (Botschantzev Citation1954; Sukhorukov Citation2014);
A. benthamii Paulsen ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. leiostachya (Benth.) K.A.Sheph. & Paul G.Wilson (K.A.Shepherd in herb. PERTH);
A. bidens Nees ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. bidens (Nees) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. brachystachyum Paulsen ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. leiostachya (Benth.) K.A. Sheph. & Paul G. Wilson (P.G.Wilson in herb. NSW);
A. capense Moss ≡ Sarcocornia capensis (Moss) A.J. Scott (Steffen et al. Citation2010);
A. caspicum (Pall.) Moq. nom. illegit. (invalid basyonym: Salicornia caspica Pall. 1771 non L. 1753) ≡ Halostachys belangeriana (Moq.) Botsch. (Sukhorukov, in present article);
A. ciliolatum Bunge ex Ung.-Sternb. ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. ciliolata (Bunge ex Ung.-Sternb.) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. coralloides Loscos & J.Pardo ≡ Microcnemum coralloides (Loscos & J. Pardo) Buen (Molero Citation1986);
A. decumbens Toelken ≡ Sarcocornia decumbens (Toelken) A.J. Scott (Steffen et al. Citation2010);
A. donaldsonii (Ewart & Jean White) C.A. Gardner ≡ Tecticornia tenuis (Benth.) K.A. Sheph. & Paul G. Wilson (P.G. Wilson in herb. MEL);
A. dunense Moss ≡ Sarcocornia dunensis (Moss) S. Steffen, Mucina & G. Kadereit (Steffen et al. Citation2010);
A. fruticosum (L.) Moq. ≡ Sarcocornia fruticosa (L.) A.J. Scott (Steffen et al. Citation2015);
A. fruticosum var. californicum Moq. ≡ see comments under A. subterminale (Sukhorukov, in present study);
A. glaucum (Delile) Ung.-Sternb. nom. illegit. (Salicornia glauca Delile 1813 non S. glauca Stokes 1812) ≡ A. macrostachyum (Moric.) K.Koch (e.g. Castroviejo Citation1990);
A. halocnemoides Nees ≡ Tecticornia halocnemoides (Nees) K.A. Sheph. & Paul G. Wilson subsp. halocnemoides (Shepherd and Wilson Citation2007);
A. halocnemoides Nees var. pergranulatum J.M.Black ≡ Tecticornia pergranulata (Nees) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. halocnemoides Nees var. pterygospermum J.M.Black ≡ Tecticornia pterygosperma (Nees) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. heptiflorum Moss ≡ Salicornia quinqueflora Bunge ex Ung.-Sternb. (Toelken Citation1967) ≡ Sarcocornia quinqueflora (Bunge ex Ung.-Sternb.) A.J. Scott;
A. hottentoticum Moss ≡ Sarcocornia pillansii (Moss) A.J. Scott (Steffen et al. Citation2010);
A. indicum (Willd.) Moq. ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. indica (Shepherd and Wilson Citation2007);
A. leiostachyum (Benth.) Paulsen ≡ Tecticornia indica (Willd.) K.A. Sheph. & Paul G. Wilson subsp. leiostachya (Benth.) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. littoreum Moss ≡ Sarcocornia littorea (Moss) A.J. Scott (Steffen et al. Citation2010);
A. lylei (Ewart & Jean White) J.M. Black ≡ Tecticornia lylei (Ewart & Jean White) K.A.Sheph. & Paul G.Wilson (Shepherd and Wilson Citation2007);
A. mossianum Toelken ≡ Sarcocornia mossiana (Toelken) A.J. Scott (Steffen et al. Citation2010);
A. namaquense Moss ≡ Sarcocornia pillansii (Moss) A.J. Scott (Steffen et al. Citation2010);
A. natalense (Bunge ex Ung.-Sternb.) Moss ≡ Sarcocornia natalensis (Bunge ex Ung.-Sternb.) A.J. Scott ((Steffen et al. Citation2010);
A. natalense var. affine (Moss) Toelken ≡ Sarcocornia natalensis (Bunge ex Ung.-Sternb.) A.J. Scott subsp. affinis (Moss) S. Steffen, Mucina & G. Kadereit (Steffen et al. Citation2010);
A. pachystachyum (Bunge ex Ung.-Sternb.) A. Chev. ≡ Salicornia pachystachya Bunge ex Ung.-Sternb. (Brenan Citation1954b);
A. perenne (Mill.) Moss ≡ Sarcocornia perennis (Mill.) A.J. Scott (de la Fuente et al. 2013);
A. pillansii Moss ≡ Sarcocornia pillansii (Moss) A.J. Scott (Steffen et al. Citation2010);
A. pillansii var. dunense (Moss) Toelken ≡ Sarcocornia dunensis (Moss) S. Steffen, Mucina & G. Kadereit (Steffen et al. 2010);
A. pruinosum Paulsen ≡ Tecticornia pruinosa (Paulsen) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. radicans (Guss.) K. Koch nom. illegit. (bas.: Salicornia radicans Guss. (1832) non Smith (1807)) ≡ Sarcocornia ?perennis (Mill.) A.J.Scott;
A. subterminale (Parish) Standl. ≡ more related to Salicornia/Sarcocornia group, but has distinct characters in the reproductive sphere in reference to this group or Arthrocnemum (Sukhorukov, present study);
A. terminale Toelken ≡ Sarcocornia terminalis (Toelken) A.J. Scott (Steffen et al. Citation2010);
A. triandrum F.Muell. ≡ Tecticornia triandra (F.Muell.) K.A. Sheph. & Paul G. Wilson (Shepherd and Wilson Citation2007);
A. variiflorum Moss ≡ Sarcocornia sp. (putative hybrid of Sarcocornia tegetaria and another species: Steffen et al. Citation2010);
Arthrocnemum virginicum (Forskål) Fritsch nomen illegit. ≡ Salicornia virginica Forskål (1775) nom. illegit. non L. (1753);
A. xerophilum Toelken ≡ Sarcocornia xerophila (Toelken) A.J. Scott (Steffen et al. Citation2010).
Conclusion
The genus Arthrocnemum in its recent circumscription is a mostly Mediterranean and West African genus of two representatives, with no species occurring in Australia or America. Despite the similarities in morphology and habitat of Arthrocnemum and the Salicornia/Sarcocornia group, the most important differences between them are found in anatomical characters, especially some involving the reproductive organs and which can be used in studies of other Salicornioideae.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The study was carried out with the support of grants from the Russian Fund for Basic Research (revision of the herbaria in UK: project 14-04-00136-a), Russian Science Foundation (carpological research: 14-50-00029), and project of the Department of Higher plants, Moscow State University (revision of the herbaria in Moscow: AAAA-A16-116021660045-2).
Notes on Contributors
Alexander P. Sukhorukov, Dr. Sci. since 2016, Leading Scientist at the Department of Higher Plants, Biological Faculty (Moscow Lomonosov State University), Professor of Chinese Academy of Sciences (since 2012), Member of the Russian Botanical Society and Botanical Society of America. Main research fields: flora of Russia; flora of Himalaya; flora of Africa; anatomy; taxonomic, carpological and molecular revisions of Chenopodiaceae, Molluginaceae, Nyctaginaceae (all – Caryophyllales), and Asteraceae. Author or co-author of the taxonomic treatments of some families in the manual identifications of European Russia and Caucasus, as well as Flora of China, Flora of Iraq, Flora of Palestine, Flora of Central Africa projects. Contribution: field study; research planning; conducted anatomical studies; analysing the results and wrote the manuscript.
Maya V. Nilova, PhD, scientific staff at the Department of Higher Plants (since 2015), Biological Faculty (Moscow Lomonosov State University), Member of the Russian Botanical Society. Main research interests: plant anatomy, forensic botany. Contribution: anatomical investigations; preparing the figures.
Acknowledgements
The authors thank the reviewers Kelly A. Shepherd and Gudrun Kadereit who provided valuable comments to the previous version of the manuscript, as well as Elizabeth Dodinet, Valéry Malécot, Geoffrey Harper, Alexander Sennikov, Alexandra Konstantinova, Charo Noya Santos, Mauro Iberite and Lorenzo Cecchi for discussion of the present investigation.
References
- Aellen, P., J. Cullen, and J. E. Coode. 1967. “Arthrocnemum.” In Flora of Turkey and the East Aegean Islands, 2, edited by P. H. Davis, 320–321. Edinburgh: University Press.
- Alonso, M. A., and M. B. Crespo. 2008. “Taxonomic and nomenclatural notes on South American taxa of Sarcocornia (Chenopodiaceae).” Annales Botanici Fennici 45: 241–254.
- Baker, J. G., and C. B. Clarke. 1913. “Chenopodiaceae.” In Flora of Tropical Africa, 6(1), edited by W. T. Thiselton-Dyer, 76–94. London: Reeve & Co.
- Ball, P. W. 1964. “Arthrocnemum.” In Flora Europaea, 1, edited by T. G. Tutin, V. H. Heywood, N. A. Burges, D. H. Valentine, S. M. Walters, and D. A. Webb, 101. Cambridge: University Press.
- Ball, P. W. 2003. “Arthrocnemum.” In Flora of North America, North of Mexico, 4, edited by Flora of North America Editorial Committee, 381–382. New York & Oxford: Oxford University Press.
- Botschantzev, V. P. 1954. “Critical notes on Chenopodiaceae (I).” Botanicheskie materialy Gerbariya Botanicheskogo Instituta Akademii Nauk SSSR 16: 84–85.
- Brenan, J. P. M. 1954a. “Chenopodiaceae.” In Flora of West Tropical Africa, 1(1), edited by W. J. Keay, 143–145. London: Millbank.
- Brenan, J. P. M. 1954b. “Chenopodiaceae.” In Flora of Tropical East Africa, Chenopodiaceae, edited by W. B. Turrill and E. Milne-Readhead, 1–25. London: Crown Agents.
- Brown, G., and B. A. Mies. 2012. Vegetation ecology of Socotra. Dordrecht et al.: Springer Verlag.
- Bunge, A. 1856. “Recensio Salsolearum nonnullarum in primis a Car. Koch distinctarum herbarii regii Berolinensis.” Linnaea 28: 572–576.
- Castroviejo, S. 1990. Flora Iberica, 2. Madrid: Consejo Superior de Investigaciones Cientificas.
- Castroviejo, S., and P. Coello. 1980. “Datos cariólogicos y taxonómicos sobre las Salicorniinae A. J. Scott Ibéricas.” [“Caryological and taxonomic data on Iberian Salicorniinae A. J.Scott”] Anales del Jardín Botánico de Madrid 37(1): 41–73.
- Dangeard, P. A. 1887. “Recherches sur le structure des Salicornieae et Salsolaceae.” Bulletin de la Société linnéenne de Normandie ser. 4 (2): 88–95.
- Davy, A. J., G. F. Bishop, H. Mossman, S. Redondo-Gómez, J. M. Castillo, E. M. Castellanos, T. Luque, and M. E. Figueroa. 2006. “Biological flora of the British Isles: Sarcocornia perennis (Miller) A. J. Scott.” Journal of Ecology 94: 1035–1048.
- De Fraine, E. 1913. “The anatomy of the genus Salicornia.” Journal of the Linnean Society of London (Botany) 41: 317–348.
- De la Fuente, V., M. Oggerin, L. Rufo, N. Rodríguez, E. Ortuñez, D. Sánchez-Mata, and R. Amils. 2013. “A micromorphological and phylogenetic study of Sarcocornia A.J. Scott (Chenopodiaceae) on the Iberian Peninsula.” Plant Biosystems 147 (1): 158–173.
- Ferguson, I. K. 1964. “Notes on the stigma morphology and flowering behavior in British Salicornieae.” Watsonia 6 (1): 25–27.
- Friis, I., and M. G. Gilbert. 1993. “Chenopodiaceae.” In Flora of Somalia, 1, edited M. Thulin, 127–140. London: Royal Botanical Gardens Kew.
- Greuter, W., H. M. Burdet, and G. Long. 1984. Med-Checklist, 1. Geneva & Berlin: Conservatoire & Jardin botaniques de la Ville de Genève & Botanischer Garten u. Botanisches Museum Berlin-Dahlem.
- Grigore, M.-N., L. Ivanescu, and C. Toma. 2014. Halophytes: an integrative anatomical study. Heidelberg et al.: Springer Int.
- Guilló, A., M. A. Alonso, and A. Juan. 2013. “New insights into seminal and stomatal morphology and their contribution to the taxonomy of the Old World succulent perennial Salicornioideae.” Plant Systematics and Evolution 299: 1185–1203.
- Guilló, A., M. A. Alonso, M. L. Lendínez, C. Salazar, and A. Juan. 2014. “Taxonomical identity of Sarcocornia fruticosa and S. hispanica in the Iberian Peninsula.” Anales del Jardín Botánico de Madrid 71 (2): e011.
- Hedge, I. 1997. “Arthrocnemum.” In Flora des Iranischen Hochlandes und der umrahmenden Gebirge (Flora Iranica), 172, edited by K. H. Rechinger, 128–130. Graz: Akademische Druck- u. Verlagsanstalt.
- Hiern, W. P., A. B. Rendle, and F. Welwitsch. 1896. Catalogue of the African plants collected by Dr. Friedrich Welwitsch in 1853-61, 1(4). London: printed by order of the Trustees.
- ICN (International Code of Nomenclature for algae, fungi, and plants [Melbourne code]). 2012. Melbourne: ICN.
- IUCN. 2014. Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee. Available from http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed: 15 May 2015).
- Jalas, J. and J. Suominen. 1980. Atlas Florae Europaeae, 5. Helsinki: The Committee for mapping the flora of Europe and Societas Biologica Fennica.
- Jafri, S. M. H., and F. B. Rateeb. 1978. “Chenopodiaceae.” In Flora of Libya, 58, edited by S. M. H. Jafri and A. El-Gadi, 1–109. Tripoli: Al Faateh University.
- Kadereit, G., and A. E. Yaprak. 2008. “Microcnemum coralloides (Chenopodiaceae-Salicornioideae): an example of intraspecific East-West disjunctions in the Mediterranean region.” Anales del Jardín Botánico de Madrid 65 (2): 415–426.
- Kadereit, G., L. Mucina, and H. Freitag. 2006. “Phylogeny of Salicornioideae (Chenopodiaceae): diversification, biogeography, and evolutionary trends in leaf and flower morphology.” Taxon 55 (3): 617–642.
- Koch, K. 1853. Hortus Dendrologicus. Berlin: Schneider & Co.
- Kühn, U. (with additions by V. Bittrich, R. Carolin, H. Freitag, I. C. Hedge, P. Uotila, and P. G. Wilson). 1993. “Chenopodiaceae.” In The families and genera of vascular plants, 2: Flowering Plants, Dicotyledons: Magnoliid, Hamamelid and Caryophyllid families, edited by K. Kubitzki, J. G. Rohwer and V. Bittrich, 253–281. New York, NY: Springer.
- Lagasca, M. 1817. Memoria sobre las plantas barrilleras de España [Memoires about alkaline plants of Spain]. Madrid: La imprenta real.
- Lebrun, J.-P., and A. L. Stork. 1991. Enumeration des plantes a fleurs d’Afrique Tropicale, 1. Geneva: Conservatiore et Jardin botaniques de la Ville de Genéve.
- Lebrun, J.-P., and A. L. Stork. 2003. “Tropical African flowering plants. Ecology and distribution, 1.” Geneva: Conservatoire et Jardin botaniques de la Ville de Genéve.
- Martins, E. S. 2002. “Flora de Capo Verde (plantas vasculares). Chenopodiaceae, 14.” Lisboa–Praia: Imprensa de Coimbra.
- Meikle, R. D. 1985. Flora of Cyprus, 2. Kew: The Bentham-Moxon-Trust.
- Maire, R. 1962. Flore de l’Afrique du Nord, 4. Paris: Léchevalier.
- Milič, D., J. Lukovič, M. Dan, L. Zorič, D. Obreht, S. Veselič, G. Anačkov, and T. Petanidou. 2011. “Identification of Salicornia population: anatomical characterization and RAPD fingerprinting”.” Archives of Biological Science (Belgrade) 63 (4): 1087–1098.
- Molero, J. 1986. “Taxonomía del género Microcnemum Ung. Sternb.” [Taxomony of the genus Microcnemum Ung. Sternb.] Collectanea Botanica 16: 327–336.
- Moquin-Tandon, A. 1840. Chenopodearum monographica enumeratio. Paris: Loss Typ.
- Moris F.-H. and F. Delporte 1854. “Enumeratio seminum regii horti botanici Taurinensis.” Annales des Sciences Naturelles (Botanique), ser. 4, 2: 377.
- Moss, C. E. 1954. “The species of Arthrocnemum and Salicornia in Southern Africa.” Journal of South African Botany 20 (1): 1–22.
- O’Callaghan, M. 1992. “The ecology and identification of the southern African Salicornieae (Chenopodiaceae).” South African Journal of Botany 58 (6): 430–439.
- O’Callaghan, M., and E. G. H. Oliver. 1992. “Transfer of Arthrocnemum varieties to Sarcocornia (Chenopodiaceae).” South African Journal of Botany 58 (6): 540.
- Parish, S. B. 1898. “New or little-known plants of Southern California I.” Erythea 6: 85–92.
- Quattrocchi, U. 2000. CRC World dictionary of plant names, 4. Boca Raton–New York–Washington: CRC Press.
- Romeiras, M. M., S. Catarino, I. Gomes, C. Fernandes, J. C. Costa, J. Cauapé-Costells, and M. C. Duarte. 2016. “IUCN Red List assessment of the Cape Verde endemic flora: towards a global strategy for plant conservation in Macaronesia.” Botanical Journal of the Linnean Society 180 (3): 413–425.
- Rouy, G. 1910. Flore de France; ou, Description des plantes qui croissent spontanément en France, en Corse et en Alsace-Lorraine [Flora of France; description of the native plants growing in France, Corsica and Alsace] Paris: Deyrolle.
- Schmidt, J. A. 1852. Beiträge zur Flora der Cap Verdischen Inseln: mit Berücksichtigung aller bis jetzt daselbst bekannten wildwachsenden und kultivierten Pflanzen: nach eigenen Untersuchungen und mit Benutzung der gewonnenen Resultate anderer Reisenden [Additions to the flora of Cape Verde Islands, with a consideration of all known native and cultivated plants based on the own investigations and results of other travelers]. Heidelberg: E. Mohr.
- Scott, A. J. 1977. “Reinstatement and revision of Salicorniaceae J. Agardh (Caryophyllales).” Botanical Journal of the Linnean Society 75 (4): 357–374.
- Sennen, F. 1936. “Diagnoses des nouveautés parues dans les exsiccata. Plantes d’Espagne et du Maroc, de 1928 à 1935.” [with no information about publishing place and house].
- Shepherd, K. A., M. Waycott, and A. Calladine. 2004. “Radiation of the Australian Salicornioideae (Chenopodiaceae) – based on evidence from nuclear and chloroplast DNA sequences.” American Journal of Botany 91 (9): 1387–1397.
- Shepherd, K. A., T. D. Macfarlane, and T. D. Colmer. 2005a. “Morphology, anatomy and histochemistry of Salicornioideae (Chenopodiaceae). Fruits and seeds.” Annals of Botany 95: 917–933.
- Shepherd, K. A., T. D. Macfarlane, and M. Waycott. 2005b. “Phylogenetic analysis of the Australian Salicornioideae (Chenopodiaceae) based on morphology and nuclear DNA.” Australian Systematic Botany 18 (1): 89–115.
- Shepherd, K. A. 2007. “Three new species of Tecticornia (formerly Halosarcia) (Chenopodiaceae: Salicornioideae) from the Eremaean botanical province, Western Australia.” Nuytsia 17: 353–366.
- Shepherd, K. A., and P. G. Wilson. 2007. “Incorporation of the Australian genera Halosarcia, Pachycornia, Sclerostegia and Tegicornia in Tecticornia (Salicornioideae, Chenopodiaceae).” Australian Systematic Botany 20 (4): 319–331.
- Standley, P. C. 1914. “The genus Arthrocnemum in North America.” Journal of the Washington Academy of Sciences 4: 398–399.
- Steffen, S., L. Mucina, and G. Kadereit. 2009. “Three new species of Sarcocornia (Chenopodiaceae) from South Africa.” Kew Bulletin 64: 447–459.
- Steffen, S., L. Mucina, and G. Kadereit. 2010. “Revision of Sarcocornia (Chenopodiaceae) in South Africa, Namibia and Mozambique.” Systematic Botany 35 (2): 390–408.
- Steffen, S., P. Ball, L. Mucina, and G. Kadereit. 2015. “Phylogeny, biogeography and ecological diversification of Sarcocornia (Salicornioideae, Amaranthaceae).” Annals of Botany 115 (3): 353–368.
- Sukhorukov, A. P. 2008. “Fruit anatomy of Anabasis (Salsoloideae, Chenopodiaceae).” Australian Systematic Botany 21 (6): 431–442.
- Sukhorukov, A. P. 2010. “Heterodiaspory and its correlation with heteroanthocarpy and fruit/seed dimorphisms (a case study of some Chenopodiaceae representatives).” Byulleten’ Moskovskogo Obshchestva Ispytatelei Prirody, Otdel Biologicheskiy 115: 39–47.
- Sukhorukov, A. P. 2014. The carpology of the Chenopodiaceae with reference to the phylogeny, systematics and diagnostics of its representatives. Tula: Grif & Co. . (in Russian with English summary).
- Sukhorukov, A. P., E. V. Mavrodiev, M. Struwig, M. V. Nilova, K. K. Dzhalilova, S. A. Balandin, A. Erst, and A. A. Krinitsyna. 2015. “One-seeded fruits in the core Caryophyllales: their origin and structural diversity.” Plos One 10 (2): e0117974.
- Thiers, B. (2008+) [ continuously updated] “Index herbariorum: A global directory of public herbaria and associated staff.” New York: Botanical Garden. Available from: http://sweetgum.nybg.org/ih/ (accessed: 20 June 2013).
- Toelken, H. R. 1967. “The species of Arthrocnemum and Salicornia (Chenopodiaceae) in Southern Africa.” Bothalia 9 (2): 255–307.
- Ungern-Sternberg, F. 1866. Versuch einer Systematik der Salicornieen [An attempt of the Salicornieae revision]. Dorpat: E.J. Karow.
- Ungern-Sternberg, F. 1876. “Salicorniearum synopsis.” Atti Congresso Internazionale Botanico tenuto a Firenze, edited by Tuscanian Society of Horticulture, 259–343. Firenze: M. Ricci.
- Volkens, G. 1893. “Chenopodiaceae. ” In Die Natürlichen Pflanzenfamilien, 3, 1a, edited by A. Engler, and K. Prantl, 36–91. Leipzig: Verlag von Wilhelm Engelmann.
- White, F. 1983. The vegetation of Africa. Published by UNESCO.
- Wilder, B. T., R. S. Felger, and H. Romero-Morales. 2008. “Succulent plant diversity of the Sonoran Islands, Gulf of California, Mexico.” Haseltonia 14: 127–160.
- Wilson, P. G. 1980. “A revision of the Australian species of Salicornieae (Chenopodiaceae).” Nuytsia 3 (1): 3–154.
- Yaprak, A. E. 2008. Taxonomic revision of the genera Salicornia, Sarcocornia and Arthrocnemum (Chenopodiaceae) in Turkey. Ph.D. Thesis. Ankara: Ankara University.
- Zare, G., and M. Keshavarzi. 2007. “Morphological study of Salicornieae (Chenopodiaceae) native to Iran.” Pakistan Journal of Botany 10 (6): 852–860.
- Zohary, M. 1966. Flora Palaestina, 1. Jerusalem: The Israeli Academy of Sciences and Humanities.
Appendix 1.
Specimens studied of Arthrocnemum macrostachyum and Arthrocnemum subterminale for purposes of comparison
Arthrocnemum macrostachyum: [Israel/Jordan] Mer Morte [Dead Sea] [without date] Tenore s.n. (LE); [Italy, Sicily] Trapani, September 1900, H. Ross 273 (LE); Israel, Dead Sea, 27 May 1902, J.E. Dinsmore 9102 (HUJ); [France] Rhone-Mündung, August 1951, Markgraf s.n. (B); [Spain] Almeria, 22 August 1982, G. Kunkel 19855 (B); Egypt, Burgh-el-Arab, 27 September 1987, H. Freitag 19688 (LE); Cyprus, Larnaca, November 2006, A. Sukhorukov s.n. (MW);
A. subterminale (a distinct taxon not referring to Arthrocnemum after present investigation): USA, California, Carpenteria, 11 August 1965, R.F. Thorne & P. Everett 35349 (BM); USA, California, San Diego co., city of San Diego, 9 July 2007, L. Morgan 0235 (K).