In this series of ‘Letters to the twenty-first century botanist’ dedicated to the flower (Dodinet and Selosse Citation2016), Nadot and Dodinet (Citation2016) established a first definition based on morphology and supported by evo-devo. Dodinet (Citation2016) then challenged this view by proposing the ethnobotanist’s perspective, and evidencing the variability of perception of the flower depending on the ethnographical context. But even in the framework of western science, not every scientific community sees the flower in the same way. The current essay seeks to bring an evolutionist’s outlook to the multiple ways of approaching and defining the flower. I first describe the flower in co-evolution with animals, entailing an acceleration of evolution by an arms race; then I show that the diversity of flowers provided tools for classification, especially for Linnaeus’s scheme, fully based on flowers; finally, I describe how a phenomenon arising from fast co-evolution, namely convergence, makes flower shapes often irrelevant as criteria for families retrieved by modern, phylogenetic classifications.
Flower as a battlefield
About 80% of plants are pollinated by animals (Abrol Citation2012), so the flower is involved in an interaction with animals such as insects, birds or mammals in the vast majority of flowering plants. A naive view is that of a perfect matching and complementarity between animals, seeking food or resting or even breeding sites, and flowers, which ensure out-crossing thanks to animal vectors (Figure A). Indeed, beyond morphological diversity, the functional analysis of flowers reveals various ways that allow, and often optimize, the interaction with animals (Proctor, Yeo and Lack Citation1996). Yet this is a story of winners because many protagonists of plant–animal floral interactions probably went extinct in the past, and the feeling of an optimization as observed nowadays emerges from a pile of dead, counter-selected and less optimal interactions that may have existed in the past.
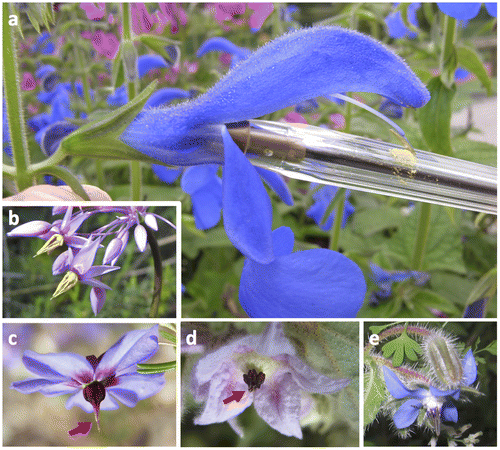
Figure 1. Flowers as manipulators of pollinators. (A) Salvia ssp. flowers illustrate a mechanism adapted to pollinator behaviour, where access to the nectar, prevented on the lateral sides by fused corolla and calix, forces the animal (here modelled by a pen) to act on a lever mechanism that bends the two stamens, connected together at their midpoint, onto the back of insects, or onto the beak of birds. (B–E) Buzz-pollinated, salt-shaker-like flowers from unrelated families, respectively (B) Sowerbaea laxiflora (Asparagaceae), (C) Platytheca galioides (Elaeocarpaceae), (D) Thomasia macrocarpa (Malvaceae) and (E) Borago officinalis (Boraginaceae). Red arrows: poricidal opening.
Animal-pollinated flowers combine three kinds of features. The first feature, relevant for plant reproduction, is production of pollen, which must be deposited on or harvested from the animal vectors. The second feature is relevant on the animal side: it is the reward, most often a nutritional one, but sometimes a shelter. The third and last feature allows animal attraction, and concerns flower shape, size, colour, odour and timing of anthesis (Faegri and Van der Pijl Citation1979), or even its surface microstructure (Whitney et al. Citation2009). Yet these features are not necessarily linked to each other: the reward (most of the time, sugar) is not conspicuous or attractive in itself as it has no colour or odour so that flowers are attractive by aposematism, i.e. only after animals are selected by floral features, or gain experience with the signals displayed; pollination is independent of the reward for the animal, which allows cheating (animals can pollinate without reward, or take the reward without pollinating). Indeed, this allows evolutionary conflicts, where one partner does not pay the reward linked to the interaction, such as nectar-free, but attractive flowers (for instance, 30% of orchid species are rewardless; Jersakova, Johnson and Jürgens, 2009; Selosse Citation2014) or non-pollinating animals that rob nectar (Inouye Citation1983). Although the impact of cheaters on fitness (= number of offspring) of the partners is variable (e.g. Maloof and Inouye Citation2000), such cheaters pave the way for the emergence of truly parasitic species (Sachs and Simms Citation2006). Moreover, floral cheating repeatedly evolves (e.g. Chartier, Gibernau and Renner Citation2014).
Hence, many flower features can be viewed as secondarily selected to enforce animal cooperation, as a result of co-evolution with pollinators and cheater avoidance. For example, in Salvia spp. flowers, the fused corolla and calix as well as the stamen with a lever mechanism (Figure A) all force animals to receive pollen on their way to nectar, situated at the base of the corolla tube. Rather than a marvellous complementarity with animal partners, the zoogamous flower is a battlefield where only the survivors, more or less adapted to the partners, and more or less avoiding cheaters, can be observed. Tomorrow, a new trait in one species may promote the emergence of new cheaters, or better cheating avoidance. Flowers have to adapt to both abiotic and biotic changes, the latter being a selective force that, according to the Red Queen hypothesis (Van Valen Citation1973), accelerates evolutionary rates because the partners’ evolution proceeds faster than abiotic environmental changes. In other words, flowers may evolve faster, especially in terms of morphology, than other organs because of an evolutionary arms race with pollinators. Flowers track the evolution of their partners in a continuous co-evolution, but can also shift to new partners (Whittle and Hodges Citation2007).
Flower diversity and Linnaeus’s classification
Floral shapes are more diverse than, for example, the shapes of leaves, which are less directly involved in biotic interactions, and this may result in a faster evolutionary exploration of possible shapes and functioning. This is precisely where Linnaeus was efficient: he based his classification in the Systema naturae (Linnaeus Citation1753, Citation1758; Figure ) on flowers that vary a lot and provide many criteria due to their complexity. Although most authors before him used floral criteria for classification, his system is exclusively based on flowers (Figure ); moreover, it is also based on a seductive interpretation of the flower, derived from precursors such as Camerarius and Tournefort (Vallade Citation2008), which survives today and made his classification popular: the flower is the place for mating, hence the name ‘sexual system’ for Linnaeus’s classification. Nowadays, in a different exercise, plant identification, we still use flowers (with few exceptions, see Eggenberg and Mohl Citation2013), a fact probably explained by both the intrinsic variability of flowers and an inheritance of Linnaeus’s emphasis on flowers. Yet, purely in terms of classification, a close look at Figure reveals how our current view of classification has changed. Linnaeus distinguished flowers where sexuality is invisible (Clandestinae) versus visible (Publicae). In the latter, Diclina have unisexual flowers while Monoclina have hermaphroditic flowers with male and female parts on the thalamus that are either close (Affinitas) or separate (Diffinitas). Within the latter, one section is subdivided on the basis of the number of stamens (Mon-, Di-, Triandria, etc.; Figure ).
Figure 2. Linnaeus’s classification of plants: the ‘Key to the sexual system’ from the 10th edition of Systema Naturae (Linnaeus, 1758).
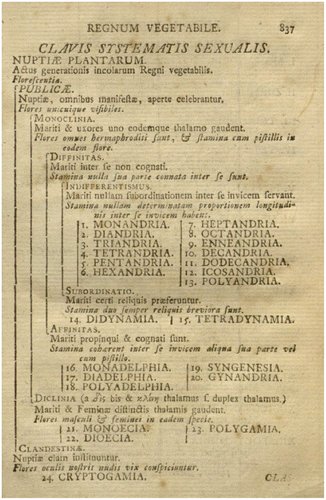
This emphasizes the outstanding contribution of flowers to the eighteenth century classification, but also how things have changed since then, as Linnaeus’s classification of plants did not survive. Indeed, at that time, classification was rather a description of what God created, in a fixist view of Nature, as shown in Linnaeus’s words Deus creavit, Linnaeus disposuit (“God created, Linnaeus organized”). Nowadays, classifications take into account a fundamental characteristic of living beings: evolution. Having evolved and sharing more or less ancient common ancestors with other species, living beings can be classified according to this specific property, which creates various levels of relatedness (Selosse and Durrieu Citation2004). The so-called phylogenetic system is best exemplified by the work of the ‘Angiosperm Phylogeny Group’ (APG III Citation2009; APG IV Citation2016). Not only are more diverse characters (predominantly molecular) now taken into account, but also the evolutionary status of traits, derived (apomorphic) or ancestral (plesiomorphic), is a post hoc product of the analysis (see discussion in Selosse and Durrieu Citation2004). Many categories homogeneous in the Systema naturae turned out to be polyphyletic, because they relied on traits that emerged repeatedly. For example, unisexual flowers evolved independently thousands of times (Renner Citation2016). Ironically, this system, which was the big disponendum target of Linnaeus, is now forgotten, whereas we have retained the binomial naming of species, names that were trivial in his eyes, entirely factitious and good only until specific essential names could be established (Selosse Citation2011).
To be fair, let us say that Linnaeus himself considered that his system was artificial, and that ‘artificial systems are entirely necessary as long as we lack a natural one’ (Linnaeus 1735). Yet the importance of flowers in classifications survived Linnaeus and the eighteenth century, together with the fact that their role in plant sexuality often meant that their role in biotic interaction was seen as secondary, or even overlooked.
Evolutionary saturation and convergences in floral evolution
In the arms race with pollinating animals, acceleration of evolution can be expected to result in the repeated emergence of similar organizations and underlying strategies, a phenomenon called evolutionary saturation. Several traits may have undergone saturation. For example, the fusion of the corolla, or sympetaly, once used to define the Sympetalae, characterizes the so-called “Asterids”, but it is sporadically present in Monocots (e.g. some Burmanniaceae), basal eudicots (some Menispermaceae and Delphinium in Ranunculaceae), and Rosids (e.g. Crassulaceae, Malvaceae) (Endress Citation2011). As stated above, this may be interpreted as a way to drive animals to nectar by way of the sexual parts of the flower (Figure A).
Many other pollinating mechanisms arose repeatedly: the buzz-pollinated salt-shaker-like flowers present a suite of traits that evolved jointly and repeatedly (Figure. B–E). Flowers are bent on one side with more or less fused petals, which prevents any access to the underside where nectar is produced; anthers are joined together with poricidal dehiscence (opening by a terminal hole; Figure C,D), and the mature pistil emerges in the centre of the anther group. This fertile column is the place where insects hang on, but, since it offers limited gripping ability, the animals keep flying and generate vibrations that release the pollen or allow its deposition on the pistil (Proctor, Yeo and Lack Citation1996). Such flowers are known from some (but not all) Annonaceae, Asparagaceae (Figure. B), Boraginaceae (Figure E), Elaeocarpaceae (Figure C), Ericaceae (sensu lato), Malvaceae (Figure D), Primulaceae, Gesneriaceae, Solanaceae, etc. They show that several traits, at first glance independent (flower shape and bending, poricidal dehiscence), may evolve jointly within a common function, making convergence often morphologically complex.
Another highly convergent trait is the existence of multiple (often yellow) stamens, which is interpreted as a way of attracting pollen-eating pollinators such as beetles (Proctor, Yeo and Lack Citation1996): this strategy ensures that insects interact with the pollen, and aligns the reward and the pollination, but at the expense of a large loss of pollen. More generally, pollination experts recognize the so-called “pollination syndromes” (Ashworth et al. Citation2015), i.e. convergent evolution towards similar forms among various plants that target the same guild of pollinators, in a loose form of convergence.
Flowers are no longer flagships for high-level taxa
Hence, similar-looking flowers do not necessarily belong to phylogenetically related plants. First, unrelated clades may exhibit convergence; second, due to fast evolution, related species may differ in floral shape. In the examples above of sympetaly or buzz-pollinated salt-shaker-like flowers, few or no families are homogeneous for the focus floral appearance. At least in the zoogamous groups, the flower is labile and changing because it is an active co-evolutionary battlefield. Whereas the flower is a good species marker, it is not necessarily a good marker for higher taxonomic levels, e.g. the family. Although some flower types may clearly identify a given family, not all members of the family share this type of flower.
In this sense, Linnaeus’s choice to classify plants exclusively on the basis of flowers and their sexuality, although relevant in his time of fixism, did not prepare subsequent generations for a phylogenetic, evolutionary classification. Because floral characters often show poor phylogenetic conservatism due to the arms race, high-level taxa are no longer easily identifiable by flower shape. This aspect of modern phylogenetic classifications still often bothers students and their teachers. However, this is not fully new: the Ranunculaceae and Rosaceae families have long since been acknowledged without a unifying flower shape. One lesson is that most conspicuous features such as flowers, taken alone, are often misleading when defining high-level taxa. Another lesson is the need to pay more attention to vegetative criteria, which are sometimes neglected by botanists, but which may offer a better phylogenetic conservatism.
… and last but not least, coming back to a definition of flowers for the twenty-first century botanist: the flower is often a battlefield, and a place for an active, fast co-evolution with pollinators.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes on contributor
Marc-André Selosse is a professor at Muséum national d’Histoire naturelle and in universities of Gdansk (Poland) and Viçosa (Brazil). His research focuses on symbioses, and especially on the mycorrhizal symbiosis. He his involved in teaching, especially to future high school teachers, at Muséum and in many other institutions in France and worldwide. Selosse is President of the Société Botanique de France and is editor for Symbiosis, The New Phytologist and Botany Letters). His research papers and outreach essays are freely available online at http://isyeb.mnhn.fr/annuaire-etpages-personnelles/pages-personnelles/article/selosse-marc-andre.
Acknowledgements
Marc-André Selosse is supported by the Fondation Ars Cuttoli & Paul Appell, and the Polish National Science Centre (Maestro7-NZ project Orchidomics). He sincerely acknowledges P. Selosse, E. Dodinet and S. Nadot for discussions, D. Marsh for English corrections, and dedicates this paper to the memory of the late Guy Durrieu, ut in tristitia persiteat amicitiam memoriamque.
References
- Abrol, D. P. 2012. “Non bee pollinators-plant interaction.” Chap. 9 in Pollination Biology. Berlin: Springer, Berlin.
- APG III 2009. “An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. APG III.” Botanical Journal of the Linnean Society 161: 105–121.
- APG IV 2016. “An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV.” Botanical Journal of the Linnean Society 181: 1–20.
- Ashworth, L., R. Aguilar, S. Martén-Rodríguez, M. Lopezaraiza, G. Avila-Sakar, V. Rosas-Guerrero, and M. Quesada. 2015. “Pollination syndromes: a global pattern of convergent evolution driven by the most effective pollinator.” In Evolutionary biology: biodiversification from genotype to phenotype, edited by P. Pontarotti, 203–224. Marseille: Springer.
- Chartier, M., M. Gibernau, and S. S. Renner. 2014. “The evolution of pollinator–plant interaction types in the Araceae.” Evolution 68: 1533–1543.
- Dodinet, E. 2016. “Letter to the twenty-first century botanist “What is a flower?” The social science perspective.” Botany Letters 163: in press.
- Dodinet, E., and M.-A. Selosse. 2016. “Editorial.” Botany Letters 163: 1–2.
- Eggenberg, S., and A. Mohl. 2013. Flora vegetativa. Bern: Haupt Verlag.
- Endress, P. K. 2011. “Evolutionary diversification of the flowers in angiosperms.” American Journal of Botany 98: 370–396.
- Faegri, K., and L. Van der Pijl. 1979. The principles of pollination ecology. New York: Pergamon.
- Inouye, D. W. 1983. “The ecology of nectar robbing.” In The biology of nectaries, edited by T. S. Elias and B. L. Bentley, 153–173. New York: Columbia University Press.
- Jersakova, J., S.D.Johnson, and A.Jürgens. 2009. “Deceptive behaviour in plants. II. Food deception by plants: from generalized systems to specialized floral mimicry.” In Plant–-environment interactions, edited by F.Baluska, 223–246. Berlin: Springer.
- Linnaeus, C. 1735. Systema Naturae, sive Regna Tria Naturae systematice proposita per Classes, Ordines, Genera, & Species. Leiden: Haak, Leiden.
- Linnaeus, C. 1753. Species Plantarum. Stockholm: Laurentius Salvius, Stockholm.
- Linnaeus, C. 1758. Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 10th ed. Stockholm: Laurentius Salvius, Stockholm.
- Maloof, J. E., and D. W. Inouye. 2000. “Are nectar robbers cheaters or mutualists.” Ecology 81: 2651–2661.
- Nadot, S., and E. Dodinet. 2016. “Letters to the twenty-first century botanist: “What is a flower?”.” Botany Letters 163: 9–10.
- Proctor, M., P. Yeo, and A. Lack. 1996. The natural history of pollination. London: Harper Collins New Naturalist.
- Renner, S. S. 2016. “Pathways for making unisexual flowers and unisexual plants: moving beyond the “two mutations linked on one chromosome” mode.” American Journal of Botany 103: 587–589.
- Sachs, J. L., and E. L. Simms. 2006. “Pathways to mutualism breakdown.” Trends in Ecology & Evolution 21: 585–592.
- Selosse, M.-A. 2014. “The latest news from biological interactions in orchids: in love, head to toe.” New Phytologist 202: 337–340.
- Selosse, M.-A., and G. Durrieu. 2004. “Une classification mycologique phylogénétique francophone (en 2003).” Acta Botanica Gallica 151: 73–102.
- Selosse, P. 2011. “From theory of ideas to theory of succedaneum: The Linnaean botanical nomenclature(s) as a point of view on the world.” In Languages of Science in the Eighteenth Century, edited by B.-L. Gunnarsson, 157–168. Berlin/Boston: De Gruyter.
- Vallade, J. 2008. L’œil de lynx des microscopistes. Dijon: Editions Universitaires de Dijon.
- Van Valen, L. 1973. “A new evolutionary law.” Evolutionary Theory 1: 1–30.
- Whitney, H. M., L. Chittka, T. J. A. Bruce, and B. J. Glover. 2009. “Conical epidermal cells allow bees to grip flowers and increase foraging efficiency.” Current Biology 19: 948–953.
- Whittle, J. B., and S. A. Hodges. 2007. “Pollinator shifts drive increasingly long nectar spurs in columbine flowers.” Nature 447: 706–709.
