Abstract
Human activities lead to a process of homogenization of biotas in which specialist species are increasingly replaced by common and widespread species. Using a 30-year diachronic record of arable weed communities, we tested this hypothesis by quantifying changes in α- and β-diversity, using both taxonomic and functional diversity and by partitioning β-diversity into species replacement and richness differences. Arable weed communities were sampled in the same 158 fields of the Côte-d’Or region (northeastern France) between the 1970s and the 2000s. For each period, each field was characterized by crop types, soil characteristics and a High Nature Value (HNV) farmland index based on agricultural intensification at the landscape level. At the field scale, we observed a loss of 46% and 38% in α-taxonomic and functional diversity, respectively, which was in accordance with the decrease in the HNV farmland index over the same period. At the regional scale, there was an increase of 15% and 21% in β-taxonomic and functional diversity (across fields), respectively. Crop type and soil characteristics explained similar levels of variation in species replacement, and crop type explained much larger richness differences in the 2000s suggesting that crop and associated practices may exert a high filtering effect. Our results also highlighted a marked decline of common weeds; a process that is far from being counterbalanced by the few colonizing weeds. Rather than to biotic homogenization, this pattern of loss has led to a higher differentiation of arable weed communities. This could correspond to a fragmentation of suitable habitats for species that depend on weeds. This pattern was associated with a decrease of species richness per field; the loss of common species and their associated functions may be of greater significance for agroecosystem functioning.
Introduction
Intensification in agriculture, and increasingly globalized transportation, have reached an unprecedented rate that is predicted to impact species diversity and ecosystem functions at both local and large scales (Vitousek et al. Citation1997, Tilman et al. Citation2002). The negative impacts of species loss include the intrinsic value of particular taxa and potential effects on ecosystem functions (Hooper et al. Citation2005). The effect of species loss may therefore differ according to the level of functional redundancy in communities, i.e. the extent to which some species perform similar functions, and may be commutable with little impact on ecosystem processes (Rosenfeld Citation2002). The loss of a given species would have minimal impact on ecosystem processes if a “redundant” species, with a similar function, can persist. The availability of pollen and nectar resources, over the whole season, is often presented as an example with the proportion of redundant plant cover varying over time according to local flowering species composition and total richness. Although a global relationship between species richness and functional diversity has been detected (Cardinale et al. Citation2012), the loss of a given function will only occur when the last species providing this function, disappears. Therefore, there is no a priori reason to expect that changes in taxonomic and functional diversity are always linearly correlated. This underscores the importance of understanding how changes in taxonomic diversity are related to changes in functional diversity for each specific function of interest (Petchey and Gaston Citation2002).
At a regional scale, the impact of human activities has resulted in reduced habitat suitability for many endemic or ecologically specialized species and in parallel has favoured the spread of a reduced set of human-favoured species. This non-random species change is resulting in biotic homogenization (McKinney and Lockwood Citation1999), i.e. a process by which previously distinct communities become progressively more similar. At the species-level, taxonomic homogenization has now been described for many groups in several habitats including fishes (Rahel Citation2000; Scott Citation2006), birds (Lockwood, Brooks, and McKinney Citation2000; Devictor et al. Citation2007), insects (Knop Citation2016) or plants (Rooney et al. Citation2004; Smart et al. Citation2006; Vellend et al. Citation2007). Olden and Rooney (Citation2006) suggested that a broader definition of biotic homogenization should be adopted, that includes all levels of organization from the genetic, through taxonomic to functional, on which ecological processes could cause previously disparate biotas to lose biological distinctiveness. In this regard, functional homogenization can be defined as “an increase in the functional similarity of biotas over time”, associated with the establishment of species having similar “functions” in the ecosystem (e.g. high redundancy of functional traits) and the loss of species with a unique function (Olden and Rooney Citation2006). Hence, taxonomic homogenization or functional homogenization would occur when communities at different sites increasingly come to harbour the same species or traits (e.g. Fukami et al. Citation2005 for functional homogenization), i.e. when taxonomic or functional β-diversity decreased. Biotic homogenization has long been measured using classical dissimilarity indices such as Jaccard or Sørensen indices (but see Baeten et al. Citation2012) that mix two distinct phenomena (Legendre Citation2014). The first is the substitution of species in one site by different species in another site, which results in species replacement. The second is difference in species richness, that is the loss (or gain) of species at only one of the sites. New metrics have recently been developed that allow better partitioning of the two phenomena (Podani and Schmera Citation2011; Cardoso et al. Citation2014; see Materials and methods section).
Arable landscapes offer a suitable opportunity for the study of biotic homogenization. To date, no study has examined if the decline in weed species richness that followed agricultural intensification (Andreasen, Stryhn, and Streibig Citation1996; Baessler and Klotz Citation2006; Fried et al. Citation2009) has been accompanied by a decrease in weed functional diversity (but see Cardinale et al. Citation2012 for a more general overview), or by any of the two forms of biotic homogenization we describe at the regional scale. Given the role of weeds in supporting biodiversity in agroecosystems (Marshall et al. Citation2003), especially through the resources that weeds provide to pollinators (Bretagnolle and Gaba Citation2015) but also as potential reservoirs for auxiliaries, pests and pathogens (Wisler and Norris Citation2005), such a decrease in functional diversity may be of great concern for developing a more sustainable agriculture (Wezel et al. Citation2009).
Several mechanisms associated with intensive farming could have led to an increase in similarity between communities (i.e. defined here as all the weed species found in a field) and/or favoured a reduced set of response traits values within each of the communities. First, higher amounts of N-fertilization and systematic liming or drainage could have reduced differences in soil conditions across different fields. Species adapted to temporarily flooded fields (Altenfelder, Raabe, and Albrecht Citation2014), poor soils or extreme pH conditions (Richner et al. Citation2015) could have been more often lost from weed communities, whereas the most competitive and nitrophilous species could have better persisted (Fried et al. Citation2009; Fried, Chauvel, and Reboud Citation2009; Richner et al. Citation2015). Second, increased herbicide use could have selected a limited set of the less sensitive species while driving many sensitive or already rare species to local extinction (Murphy and Lemerle Citation2006). In the Côte d’Or (northeastern France), crop rotation has been modified substantially with the increase of autumn-sown crops (Fried et al. Citation2009, see also Materials and methods for further details). Hence, one might expect the remaining species to be either ecologically generalist, autumn-germinating or tolerant to herbicides, leading to a homogenization of the flora in all fields. For example, Perronne et al. (Citation2015) showed that in the 2000s, there were no functional differences between communities of different crop types. On the other hand, as different filters, among which the sowing dates or the herbicides spectrum may select different sets of species (Andersson and Milberg Citation1998) or different traits (Gunton, Petit, and Gaba Citation2011) in each particular crop, we might hypothesize that weed communities could have progressively differed (specialized) in each crop leading to higher differentiation across communities. Indeed, Fried, Chauvel, and Reboud (Citation2009, 2015) showed that, at least in sunflower and oilseed rape crops, crop mimetic species have been favoured over the last 30 years and that herbicide pressure contributed to this trend.
From the effect traits perspective, if the expected decrease of α-diversity at the local scale (within a field) is compensated by an increase of β-diversity, the maintenance of mobile organisms depending on weeds (such as pollinators) might be less affected, whereas a parallel decrease of both α- and β-diversities would certainly result in more detrimental impacts on these organisms. The aim of the present study was threefold:
| • | First, we investigated how agricultural intensification at the landscape level and resulting changes in taxonomic diversity were associated with changes in functional diversity, in terms of both response and effect traits (Lavorel and Garnier Citation2002). | ||||
| • | Second, we investigated whether species changes have led to biotic homogenization at taxonomic and/or functional levels, and how relationships among the various components of β-diversity (species replacement and differences in species richness) might explain observed changes over the last 30 years. | ||||
| • | Third, we explored how the rates of environmental homogenization, human factors (crop types) and environmental factors (soil types) explained the changes in β-diversity. | ||||
Material and methods
Data collection
The arable plant communities in 158 arable fields were studied for three successive years in the period between 1968 and 1976 (the 1970s) across the Côte-d’Or region (880,338 ha) in the continental temperate zone of northeastern France (Dessaint, Chadoeuf and Barralis Citation2001). Each year, one or two vegetation samples were performed so that between three to seven vegetation samples were available for each field in the 1970s. The same 158 fields were resurveyed in two consecutive years in 2005 and 2006 (the 2000s) with the same method. For each period, winter-sown crops were sampled once at the end of March / start of April, to account for both winter and spring annual weeds seedlings, while spring- and summer-sown crops were respectively sampled at the start of May and in June. Four crops (winter wheat, spring barley, winter barley and oilseed rape) represented > 80% of surveyed fields for both periods. Winter wheat remained the dominant crop, representing 35% and 32% of the surveyed fields in the 1970s and the 2000s, respectively. The main change in crop rotation was the abandonment of spring barley (–19%) to the benefit of winter barley (+15%) and oilseed rape (+13%). Fertilization increased steeply and then stabilized from ~30 kg/ha in the 1960s to ~60 kg/ha in the 1980s, ~160 kg/ha in the 2000s and ~140 kg/ha in 2010s (based on French Agricultural Statistical Service). In terms of weed control, just after the first period, there was an increasing availability of active ingredients against monocotyledonous weeds and the 1980–2000s period was characterized by the increase of sulphonylureas and globally by more available solutions (Chauvel et al. Citation2012)
The second survey was performed with precisely the same methods and calibrations for the weed sampling than the ones used in the 1970s. In each field, surveys were carried out in a 2000-m² plot (50 × 40 m), placed randomly in the field core (at least 20 m from the field boundaries) to avoid field-edge effects. Surveys were performed by two or more trained persons walking across the plot for a minimum of 20 min, recording all species until no new species were observed. For both surveys, all unidentified grasses and dicotyledons (that represented < 1% of the whole data set) were excluded from the analyses and some taxa were identified to genus, as identification at the seedling stage showed great discrepancy between observers. These included Adonis spp., Allium spp., Bromus spp., Carex spp., Crepis spp., Valerianella spp., Lolium spp., Rubus spp., Verbascum spp. and Vicia spp.
Each field was characterized by crop type and soil conditions. For each field, 10 soil samples had been collected in the superficial horizon (0–20 cm) within the area of the floristic surveys, and mixed into one single sample per field. The following soil variables were recorded in both periods: soil texture according to the proportion of sand, silt and clay, soil CaCO3 content, soil Cation Exchange Capacity, content of CaO, P2O5, K2O and N.
Unless otherwise stated, we analysed a combination of 2 years of weed sampling for each of the two periods. For the 2000s, we used samples from 2005 and 2006. For the surveys in the 1970s, we used the first 2 years of each 3-year survey. It should be noted that all weed data were treated as presence–absence for analysis.
α-Diversity
At the field scale, we measured species richness and functional diversity. For functional diversity, the traits were selected to optimize the representation of important functions while avoiding functionally less informative traits. Based on reference lists of traits for arable weeds (Booth and Swanton Citation2002; Gaba et al. Citation2014) we selected traits related both to responses of weeds to management practices and effects of weeds on ecosystem processes. The response traits included Raunkiaer’s life forms, maximum plant height (as recorded in floras, i.e. a rough estimate of the usual maximum height observed), seed mass, seed dispersal, and season of germination (see Table for rationale of the traits selection, their units and the sources of data). The effect traits focused on two traits (flowering phenology and pollination mode) that are supposed to influence the quality of arable fields as a suitable habitat for pollinators through the timing and amount of resources available, i.e. a community with a high proportion of insect-pollinated species flowering at different time of the year and covering all the seasons is supposed to be of higher value for the pollinating fauna. For species identified only at the genus level, the most frequent value of the trait within the genus was used (based on species occurring in arable fields only). For seed mass and season of germination, there were 1 and 23 missing values, respectively, which were replaced by the median trait value.
Table 1. Traits used to measure functional diversity within communities and across communities, i.e. functional β-diversity (Fβ).
In the present study, we were interested in how the loss of species may cause a loss of functions and we therefore used the functional diversity index developed by Cardoso et al. (Citation2014) that corresponds to the total branch length of a community tree linking all species present. A multiple correspondence analysis was performed on the “species × traits” matrix (198 species × 7 traits). All the axes were conserved and a community tree was constructed based on Euclidian distance between species in the trait multivariate space and Ward’ clustering algorithm. The functional α-diversity computed is therefore consistent with the computation of functional β-diversity (described below). Functional diversity was first computed using the whole set of traits and then separately for response and effects traits in order to pinpoint the consequences of changes in weed functional diversity for pollinators. In order to assess the robustness of the observed patterns, we also computed functional diversity using FRic (Mason et al. Citation2005), the functional richness, that corresponds to the volume of functional space occupied by the species within a community and that is expected to be less dependent on species richness than functional diversity (Mouchet et al. Citation2010). The taxonomic and functional α-diversity were compared between the 1970s and the 2000s with a Welch’s two-sample t-test to account for different variances between the two periods. In addition, Fisher’s exact tests were performed on a contingency table between species status (as extinct, stable, or new) and the seven (qualitative) traits.
β-Diversity
At the regional scale, as in most biotic homogenization studies (Olden and Rooney Citation2006), taxonomic dissimilarity among fields was first measured using a Jaccard pairwise dissimilarity index (hereafter Tβtotal), with Tβtotal = with a, the number of species common to both sites, b the number of species only found in the richest site and c, the number of species only occurring in the poorest sites. Following Podani and Schmera (Citation2011), we then decomposed the Jaccard dissimilarity index (Tβtotal) into its two components: species replacement (Tβrepl) and richness differences (Tβrich) with Tβrepl =
and Tβrich =
. Using the unified framework proposed by Cardoso et al. (Citation2014), we quantified functional β-diversity (Fβtotal) via pairwise comparisons of communities and decomposed into its functional richness differences (Fβrich) and functional replacement (Fβrepl) fractions. These calculations used the same seven traits as for functional diversity (see Table ). All these computations were performed using the package BAT (Cardoso, Rigal and Carvalho Citation2015) using the same tree (or subset) as in α-diversity.
Then, for both taxonomic and functional levels, we computed the average inter-site dissimilarities (Taxonomic and Functional βtotal, βrepl, βrich), i.e. for all the n sites i, the mean dissimilarity with all other n–1 sites. The differences in the distribution of averaged dissimilarity were compared between the 1970s and the 2000s with a Welch’s two-sample t-test to account for different variances between the two periods.
Vegetation and environment relationships
Changes in α-diversity at the field scale between the 1970s and the 2000s were compared with changes in the High Nature Value (HNV) farmland indicator during the same period. The method used for the estimation of the HNV farmland indicator relies on the calculation and combination at the “commune” scale, i.e. typically a few km2 landscape, of three components: crop diversity, degree of intensification of the farming practices (based on the level of pesticide use and the amount of fertilization according to the French Agricultural Statistical Service) and presence of landscape elements (proportion of semi-natural habitats) considered as beneficial to biodiversity (see Pointereau et al. Citation2007 for further details on the methodology). The higher the HNV indicator, the higher the expected level of biodiversity. HNV is computed at the level of a French “commune” and therefore reveals the average landscape quality at a broader spatial scale than the monitored fields. In the surveyed region, the surface of a “commune” averages 12.4 km2. For each field, we used the HNV indicator value of the “commune” where the field was located. Our study included 81 “communes” in which there were, on average, two fields.
Following Vellend et al. (Citation2007), we first evaluated how much environmental variability had changed between the two periods through its global soil characteristics. We used an aggregated value of soil conditions including factors related to changes in farming practices such as N, K and P, and factors such as soil texture and the calcium carbonate content that were expected to remain more stable. We computed Euclidean distances between samples based on the nine quantitative metrics describing soil conditions (proportion of sand, silt and clay, soil CaCO3 content, soil Cation Exchange Capacity, content of CaO, P2O5, K2O and N). Each sampled field was then characterized by its average dissimilarity with all other sampled fields during the same period. Finally this average soil condition dissimilarity was compared between the two periods with a Welch’s two-sample t-test.
Second, we explored to what extent environmental variables explain taxonomic and functional dissimilarity among fields (as well as their replacement and richness differences components) and what is the level of explained variation in the 2000s when compared with the 1970s. We also measured the part of the variation that was respectively explained by crop types and soil conditions for each period. As crop types are expected to be easier to manage than soil conditions, the outcome of the partitioning would give a rough estimation of the possibility of mitigation or restoration of the process of homogenization. We conducted a distance-based Redundancy Analysis (dbRDA) and variation partitioning separately with the 1970s and the 2000s data sets (using functions capscale and varpart in package vegan, Oksanen et al. Citation2016). Explanatory variables consisted of the soil variables and seven crop types (1, winter wheat; 2, winter barley; 3, oilseed rape; 4, spring cereals (mainly barley); 5, pea and faba beans; 6, summer crops with large inter-rows (sugarbeet, maize, soyabean and sunflower); 7, forage crops (clover, alfalfa)). These analyses were performed on each year separately (2005, 2006 and the first and second years of the survey in the 1970s). We used the proportion of constrained inertia of the dbRDA (i.e. the sum of the eigenvalues of constrained axes divided by the sum of all eigenvalues) as a measure of the proportion of variation in β-diversity that could be accounted for by these environmental variables. Then, we partitioned the variation in β-diversity according to each subset of explanatory variables (either crop types or soil conditions) controlling for the effect of the other subset. All statistical analyses were performed with R version 3.2.3. (R Core Team Citation2015) using packages BAT (Cardoso, Rigal and Carvalho, Citation2015), FactoMineR (Le, Josse and Husson, Citation2008) functional diversity (Laliberté, Legendre and Shipley, Citation2014), and vegan (Oksanen et al, Citation2016).
Results
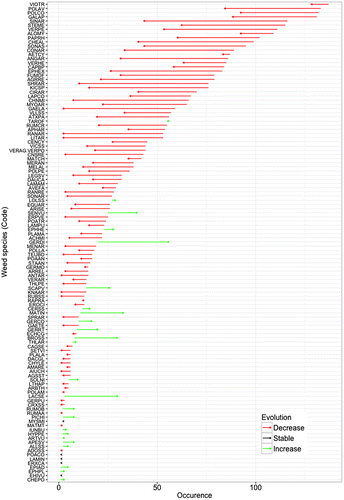
In all, 198 arable weed species were observed in the sampling, with 115 species occurring in both the 1970s and the 2000s. Forty-five and 38 species were recorded only in the 1970s and the 2000s, respectively. Five species out of the 45, which were only present in the 1970s, were observed in more than 10 fields (frequency of occurrence > 6%, e.g. Lathyrus tuberosus (39%) and Cerastium arvense (13%)) whereas only two of the 38 species that were only present in the 2000s were found in more than 10 fields (e.g. Sisymbrium officinale (9%) and Cirsium vulgare (8%)). Among the species found in both surveys, 75% (86 species) were less frequent and only 4% (five species) and 21% (24 species) were stable or more frequent, respectively (see Figure ). For species that have become less frequent, the number of fields where the species was no longer found in the 2000s was 23 (ranging from 1 to 73). For species that have become more frequent, the average number of fields where the species was only found in the 2000s was 9 (ranging from 1 to 36).
Figure 1. Changes in species frequency between the 1970s and the 2000s. Decreasing species (red arrow), increasing species (green arrow), stable species (black arrow). Species name is abbreviated using EPPO Codes, see EPPO 2016. EPPO Global Database (available online) https://gd.eppo.int.
Dramatic decline of α-diversity between 1970s and the 2000s
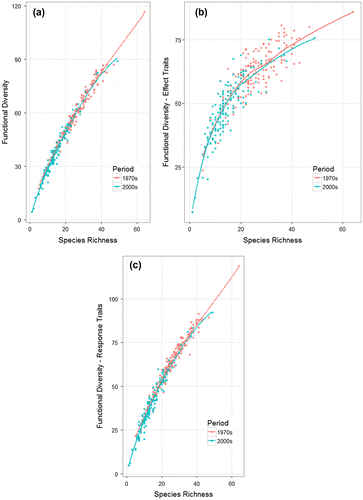
At the field scale, average species richness decreased significantly from 27 ± 8 (mean ± SD) to 15 ± 7 (Figure a, p < 0.001) between the 1970s and 2000s. Over this period, the average functional diversity decreased from 61 ± 14 to 38 ± 14 (Figure b, p < 0.001). Functional diversity was strongly correlated to species richness in the 1970s (Pearson’s r correlation = 0.980, p < 0.001) and 2000s (Pearson’s r = 0.977, p < 0.001) (Figure a). Results were similar when only considering the effect traits or the response traits (Figure b, c and see Supplementary material, Appendix S1). The effect traits showed a non-linear relationship with a threshold at around 20 species below which functional diversity declined more rapidly. The same general trends were found when using FRic for computing functional diversity (see Supplementary material, Appendices S2 and S3) with more functional redundancy in effect traits in the 1970s (see Supplementary material, Appendix S3) than in the 2000s.
Figure 2. Trends in α-diversity at taxonomic and functional level between the 1970s and the 2000s. Changes (a) in species richness and in (b) functional diversity based on all seven traits (see Table ).
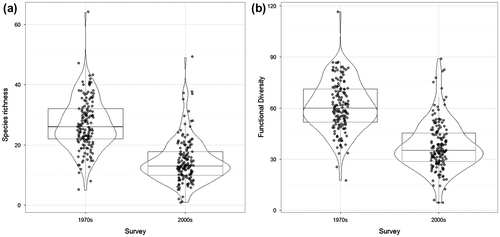
Figure 3. Relationships between species richness and functional richness in the 1970s (red dot) and in the 2000s (blue dot) based on (a) all the seven traits or only (b) on the effects traits or (c) the response traits (see Table ).
For five of the seven traits, the distribution of attributes (i.e. trait values) differed according to the change in the species frequency in time (Table ). There was an excess of extinct species among the insect-pollinated weed species and among the early-spring-flowering species. Tall and wind-pollinated species went extinct less often than expected under a random change in species frequency. Species only found in the 2000s came more frequently from among hemicryptophytes and tall species and those species showing plasticity in their season of germination.
Table 2. Traits that differ significantly according to the species status (new, stable and extinct species) and attributes more often (+) or less often (–) encountered than expected in the given status (χ2 test).
Changes in β-diversity
Taxonomic β-diversity between sites significantly increased from Tβtotal = 0.72 in the 1970s to Tβtotal = 0.83 in the 2000s (Table ). In the 1970s, the number of species shared by weed communities represented on average 39.2% of the species pool (Table ) whereas this proportion fell to 19.2% in the 2000s. On average, the species replacement component (Tβrepl) was higher by 0.28 and 0.25, respectively, than the richness differences component (Tβrich) for the 1970s and the 2000s (Table ) and contributed to a slightly higher proportion of taxonomic β-diversity in the 1970s (69%) compared with the 2000s (65%). The changes in taxonomic β-diversity between the two periods were mainly supported by higher richness differences in the 2000s (changes in Tβrich = 0.07), representing almost twice the change in species replacement (changes in Tβrepl = 0.04) (Table ).
Table 3. Mean taxonomic and functional β-diversity in the 1970s and the 2000s surveys.
Functional β-diversity increased from an average of Fβtotal = 0.56 in the 1970s to Fβtotal = 0.68 in the 2000s, following the taxonomic trend (Table ). In the 1970s, the matching component (a) of functional diversity between communities represented 77.7% of the mismatching component of functional diversity (b+c) present in only one of the community (Table ). This proportion fell to 47.4% in the 2000s. The increase in functional β-diversity between the two surveys was also supported by a greater increase in richness differences (change in Fβrich = 0.08) compared with the increase in trait replacement (change in Fβrepl = 0.03). On average, the trait replacement was higher by 0.19 and 0.14, respectively, than the richness differences component of functional β-diversity for the 1970s and the 2000s (Table ).
Vegetation and environment relationships
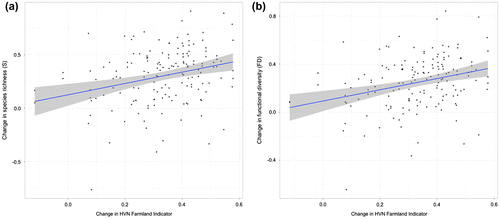
A weak but significant correlation was found between changes in α-diversity and the changes in the HNV farmland indicator (Figure ; species richness: r = 0.183, p = 0.021; functional diversity: r = 0.217, p = 0.006). The decrease in α-diversity was higher in the sites with an intense decrease in HNV farmland indicator.
Figure 4. Relationships between change in High Nature Value (HNV) farmland indicator and change in (a) species richness (Pearson’ r correlation = 0.183, P = 0.021) and (b) functional diversity (Pearson’ r correlation = 0.217, P = 0.006).
Soil conditions dissimilarity differed significantly between the 1970s and the 2000s with a narrower range of conditions observed in the 2000s (see Supplementary material, Appendix S4). The proportion of variance in taxonomic and functional β-diversity that could be related to the environment (including both crop type and soil conditions) was roughly similar in the two periods: ranging from 19.2% to 21.8% according to the subset of years (Table ).
Table 4. Comparison of explained inertia in distance-based redundancy analyses (dbRDA) performed on the distance matrix (TβTotal, Tβrepl, Tβrich, FβTotal, Fβrepl, Fβrich) in the data sets from the 1970s and the 2000s and variation partitioning between crop types (seven types) and nine soil characteristics.
Variation partitioning indicated that the proportion of species and trait replacement explained by crop type was similar (Fβrepl) or slightly higher in the 1970s (Tβrepl) whereas the proportion explained by soil conditions was always higher in the 1970s. The proportion of species and trait richness differences explained by crop types was much higher in the 2000s than in the 1970s.
Discussion
Our study showed that the loss of arable weed species between the 1970s and the 2000s was high and related to a loss of functional diversity at the field scale. Over the same time, an increase in β-diversity (between fields) was observed both at the taxonomic and functional levels, whereas the effect of environmental conditions on weed diversity remained stable, with soil conditions explaining slightly lower species replacement in the 2000s and crop types explaining more richness differences across fields.
When the response and effect traits were considered together, the global relationship between species richness and functional diversity was linear. This suggests a very low functional redundancy within weed communities, with all species having rather a unique function. This result is consistent with the patterns already observed in assemblages of birds, vertebrates, fishes and natural vegetation (Petchey and Gaston Citation2002) and suggests that under the assumption of equal importance of all of the traits used for functional diversity measurement, agroecosystem functioning would decline with any loss of biodiversity. Of course this result is dependent on the number of uncorrelated traits used to define functional diversity with more traits resulting in a more rapid decline of functional diversity (Petchey and Gaston Citation2002). Our approach of specifying in advance a restricted number of traits for which there is evidence of their importance for ecosystem functioning or of a particular function of interest (resource for pollinators in our case), allows for a clear interpretation of changes in functional diversity (see Chapin et al. Citation1996; Diaz and Cabido Citation1997).
Lower functional redundancy in weed assemblages of the 2000s
For the two effect traits related to resource for pollinators, we found a threshold in the relationships between species richness and functional diversity. This pattern was most evident when using FRic (considered to be less sensitive to species richness, Mouchet et al. Citation2010), with two clear distinct steps. At the first step, a strong decrease of species richness occurred without a similar change in functional diversity. In this range, of between 20 and 60 species, there was a high functional redundancy leading to no functional loss with the loss of species. At the second step, between 1 and 20 species, functional diversity decreased much more rapidly than species richness, which may be interpreted as the loss of species that are no longer functionally redundant. Interestingly, most of the arable weed communities in the 2000s survey were distributed across the second step of the relationship, so that missing functions were now more widespread. We can consider that ~90% of the fields no longer presented the full range of functional richness in the 2000s whereas only ~20% were concerned in the 1970s. Moreover, we found that species loss was not random. Early-flowering and insect-pollinated species went extinct more often than expected under a random loss, whereas wind-pollinated species were under-represented among the extinct species. Pinke and Gunton (Citation2014) have already observed this trend in early-flowering species and the correlation of tall species with intensive arable field management.
Together, these results indicate that the level of weed species loss between the 1970s and the 2000s reduced functional redundancy to a threshold where redundancy between weed species was no longer assured regarding pollen resource availability. This is of concern because, within a community, redundancy in response traits may ensure resilience to perturbations, whereas redundancy in effects traits may ensure a higher level of stability in ecosystem functioning (Rosenfeld Citation2002). In particular, a further species loss will induce a larger loss of pollen resource availability (e.g. communities with no insect-pollinated species, gaps in flowering period, for example in early spring). A similar difference in the proportion of insect-pollinated plants has been found when comparing conventional versus organic fields (Gabriel and Tscharntke Citation2007). Given that arable weeds provide food resources (pollen–nectar) ensuring the maintenance of honey bees (Requier et al. Citation2015) as well as wild pollinators (Rollin et al. Citation2016) and natural enemies (Nicholls and Altieri Citation2013), the level of loss in weed biodiversity observed in our study suggests a potential cascade of effects: a decrease of pollinators could directly impact agricultural production for crops that rely on pollinators. Gabriel and Tscharntke (Citation2007) suggested that the lower proportion of insect-pollinated weeds may be a consequence rather than a cause of the lower number of pollinators found in intensively managed fields. Both types of organisms have been impacted by intensive farming and it is likely that there are feedbacks between plant and pollinator diversity due to the fact that plant–pollinator communities are linked through mutualistic food webs (Gibson et al. Citation2006, Pocock, Evans, and Memmott Citation2012).
For response traits, a similar pattern was observed for functional diversity when applied to the four traits, i.e. plant height, seed mass, Specific Leaf Area and flowering onset in Mediterranean arable weed communities with a steeper decline in functional diversity and low functional redundancy at a certain level of agricultural intensification at the field scale, but not at the larger landscape scale (Guerrero et al. Citation2014). In our study, the loss of functions in the fields was in accordance with the reduction of HNV farmland indicator at the landscape level, meaning that the loss of functions in fields is not likely to be compensated by functions present in the surrounding landscape. However, as changes in species richness and functional diversity were correlated to changes in the HNV farmland indicator, this also suggests that change in practices and landscape towards more environmentally friendly management may at least partly restore previous levels of arable weeds diversity. A recent study suggested that a high proportion of organic farming at the landscape level is associated with high levels of weed species richness and especially rare arable weeds (Henckel et al. Citation2015). Another study showed that although organic farming can double the level of interesting weeds for fauna and increase by four-fold the level of rare segetal weeds, it was unable to recover the level of weed biodiversity observed before the agricultural intensification (Chamorro, Marsalles and Sans Citation2016). The effect of ecological restoration may actually depend on the fact that rare weeds are still present in the regional species pool.
Higher differentiation of weed assemblages in the 2000s
Contrary to our expectation of biotic homogenization at the regional scale due to the disappearance of specialist weeds occupying the more extreme ecological conditions (Fried, Petit, and Reboud Citation2010; Richner et al. Citation2015), our results showed that changes in arable weed communities were in fact characterized by an increase in β-diversity between the fields, both at the taxonomic and the functional trait levels. However, partitioning β-diversity showed that the increases in both taxonomic and functional β-diversity were mainly driven by the loss (or gain) of species or traits (explaining 64% and 70% of the increase of β-diversity for taxonomic and functional, respectively) although the replacement of species or functional traits remained the main components of β-diversity at both periods.
Our study confirms that soil conditions are narrower in the 2000s compared with the 1970s. However dbRDA analyses and variation partitioning showed that the relationships between weed flora and the environment characteristics remained stable, with little decrease in variation in species replacement explained by soil conditions but a strong increase in the proportion of richness differences explained by crop types. This would mean that in the 2000s, weed species assembly is less related to specific soil conditions, due for example to extinction of acidophilous species such as Gnaphalium uliginosum (extinct in eight fields), or of basiphilous species such as Iberis amara (extinct in five fields). Conversely, some specific crop types (and probably their specific practices) had a stronger filtering effect. For example, oilseed rape presented a lower average species richness than other crop types while several new colonizing species appeared almost exclusively in this crop species (Geranium dissectum, Geranium rotundifolium, Lactuca serriola). This result is consistent with previous findings made at the French national level (Fried, Chauvel, and Reboud, Citation2015).
Between 1968 and 2006 there was a general decline in weed species in France (Fried et al. Citation2009). In the present study we found that the relative importance of species local colonization was low when compared with that of local extinction processes. Only 24 species were found to increase in frequency, of which only a few (e.g. Bromus spp., Geranium dissectum, Lactuca serriola, Taraxacum sect. Ruderalia, Tripleurospermum inodorum and Senecio vulgaris) colonized more than 20 fields (about 13% of the surveyed fields). In parallel, 86 species decreased in frequency, many of which were previously common, e.g. Stellaria media, Sinapis arvensis and Veronica persica, disappearing from more than 70 fields (about 44% of the surveyed fields). This change in common species frequencies from the 1970s may explain much of the higher differentiation in arable weed communities at the regional scale in the 2000s. Some ecologically distinct, specialist species were removed in some fields, so we expected a homogenization of communities. However, this loss did not have a sufficient weight to counterbalance the local extinction of previously common and widespread species with few environmental preferences. For example, 11 species, including Elytrigia repens, Kickxia spuria and Lysimachia arvensis, went extinct from more than half of the fields. Hence, we observed higher difference in species composition that is in accordance with the predictions of Olden and Poff’s models (Citation2003, Citation2004). These important differences result in a patchwork of species-poor communities at the landscape scale that still maintains comparable species γ-diversity at the regional scale (160 species in the 1970s against 153 in the 2000s).
Conclusions
In studies of biotic homogenization, attention has mainly focused on how common and widespread species became more common and more widespread, and how specialist species with narrow ecological requirements became progressively extinct (Olden and Rooney Citation2006). Both patterns were observed in arable lands: the increasing occurrence of some invasive species, such as the native North American Ambrosia artemisiifolia (Chauvel et al. Citation2006), and the decrease of the proportion of specialist compared with generalist weed species between the 1970s and the 2000s (Fried, Petit, and Reboud Citation2010). However, our study highlighted that in the winter-cropping systems of northeastern France, the massive decline of formerly common weeds and the associated loss of functional trait attributes is a potentially much more significant process in arable fields, directly affecting ecosystem functions and many other species (Gaston and Fuller Citation2007). We also suggest that conservation tools might gain from being used to manage common arable weed species in complement to those already focusing on rare species, and so to conserve or restore these species at an acceptable level to maintain key ecosystem functions and trophic resources, such as for pollination, while limiting competition for resources.
Notes on contributors
Guillaume Fried is a weed ecologist working at the Plant Health Laboratory of Anses (French Agency for Food, Environmental and Occupational Health & Safety). Contribution: performed the 2000s floristic survey, designed and contributed to statistical analysis, drafted the manuscript.
Fabrice Dessaint is a data scientist working at INRA, Dijon with interest in plant community ecology. Contribution: contributed to statistical analysis and reviewed the manuscript.
Xavier Reboud is an agroecologist with interest in plant health; he is currently deputy head of the Plant Health and Environment Division at INRA. Xavier’s interests are on the diversity of organisms that derives from weeds’ presence and plant community response to agricultural changes. Contribution: stimulator and coordinator of a few surveys of weed species in France among which the one illustrated in this article.
Supplemental data
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/23818107.2016.1234410.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Online Material
Download MS Word (797.6 KB)Acknowledgements
We are grateful to David A. Bohan for improving a former version of the manuscript as well as to Sabrina Gaba, Richard Gunton and an anonymous reviewer who provided useful comments on this manuscript. We thank the INRA staff for their support during the weed survey of 2005–2006 and Gilbert Barralis and René Chadoeuf for conducting the initial 1970s survey. We thank Frédéric Coulon and Philippe Pointereau (Solagro) for providing the data about HNV farmland indicators in the 1970s and the 2000s.
References
- Altenfelder, S., U. Raabe, and H. Albrecht. 2014. “Effects of water regime and agricultural land use on diversity and species composition of vascular plants inhabiting temporary ponds in northeastern Germany.” Tuexenia 34: 145–162.
- Andersson, T. N., and P. Milberg. 1998. “Weed flora and the relative importance of site, crop, crop rotation, and nitrogen.” Weed Science 46: 30–38.
- Andreasen, C., H. Stryhn, and J. C. Streibig. 1996. “Decline of the flora in Danish arable fields.” Journal of Applied Ecology 33: 619–626.
- Baessler, C., and S. Klotz. 2006. “Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years.” Agriculture, Ecosystems and Environment 115: 43–50.
- Baeten, L., P. Vangansbeke, M. Hermy, G. Peterken, K. Vanhuyse, and K. Verheyen. 2012. “Distinguishing between turnover and nestedness in the quantification of biotic homogenization.” Biodiversity and Conservation 21: 1399–1409.
- Booth, B. D., and C. J. Swanton. 2002. “Assembly theory applied to weed communities.” Weed Science 50 (1): 2–13.
- Bretagnolle, V., and S. Gaba. 2015. “Weeds for bees? A review.” Agronomy for Sustainable Development 35 (3): 891–909.
- Cardinale, B. J., J. E. Duffy, A. Gonzalez, D.U. Hooper, C. Perrings, P. Venail, ... and A. P. Kinzig. 2012. “Biodiversity loss and its impact on humanity.” Nature 486(7401): 59–67.
- Cardoso, P., F. Rigal, and J. C. Carvalho. 2015. “BAT – Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity.” Methods in Ecology and Evolution 6 (2): 232–236.
- Cardoso, P., F. Rigal, J. C. Carvalho, M. Fortelius, P. A. V. Borges, J. Podani, and D. Schmera. 2014. “Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components.” Journal of Biogeography 41 (4): 749–761.
- Chamorro, L., R. M. Masalles, and F. X. Sans. 2016. “Arable weed decline in Northeast Spain: Does organic farming recover functional biodiversity?” Agriculture, Ecosystems & Environment 223: 1–9.
- Chapin, F. S. I., M. S. Bret-Harte, S. E. Hobbie, and Z. Hailan. 1996. “Plant functional types as predictors of transient responses of arctic vegetation to global change.” Journal of Vegetation Science 7: 347–358.
- Chauvel, B., F. Dessaint, C. Cardinal-Legrand, and F. Bretagnolle. 2006. “The historical spread of Ambrosia artemisiifolia L. in France from herbarium records.” Journal of Biogeography 33: 665–673.
- Chauvel, B., J. P. Guillemin, J. Gasquez, and C. Gauvrit. 2012. “History of chemical weeding from 1944 to 2011 in France: changes and evolution of herbicide molecules.” Crop Protection 42: 320–326.
- Dessaint, F., R. Chadoeuf, and G. Barralis. 2001. “Diversity of weed communities of annual crops in côte-d’Or, France.” Biotechnology, Agronomy and Society and Environment 5 (2): 91–98.
- Devictor, V., R. Julliard, D. Couvet, A. Lee, and F. Jiguet. 2007. “Functional homogenization effect of urbanization on bird communities.” Conservation Biology 21: 741–751.
- Diaz, S., and M. Cabido. 1997. “Plant functional types and ecosystem function in relation to global change.” Journal of Vegetation Science 8: 463–474.
- Fried, G., B. Chauvel, and X. Reboud. 2009. “Large-scale temporal shifts in weed assemblages analyzed by functional groups; a case study of 30-years of change in sunflower crops.” Journal of Vegetation Science 20: 49–58.
- Fried, G., B. Chauvel, and X. Reboud. 2015. “Weed flora shifts and specialisation in winter oilseed rape in France.” Weed Research 55: 514–524.
- Fried, G., S. Petit, F. Dessaint, and X. Reboud. 2009. “Arable weed decline in Northern France: crop edges as refugia for weed conservation?” Biological Conservation 142: 238–243.
- Fried, G., S. Petit, and X. Reboud. 2010. “A specialist–generalist classification of the French arable flora and its response to changes in agricultural practices.” BMC Ecology 10: 20.
- Fukami, T., T. M. Bezemer, S. R. Mortimer, and W. H. van der Putten. 2005. “Species divergence and trait convergence in an experimental community assembly.” Ecology Letters 8: 1283–1290.
- Gaba, S., G. Fried, E. Kazakou, B. Chauvel, and M. L. Navas. 2014. “Agroecological weed control using a functional approach: A review of cropping systems diversity.” Agronomy for Sustainable Development 34 (1): 103–119.
- Gabriel, D., and T. Tscharntke. 2007. “Insect pollinated plants benefit from organic farming.” Agriculture, Ecosystems and Environment 118: 43–48.
- Gaston, K. J., and R. A. Fuller. 2007. “Biodiversity and extinction: losing the common and widespread.” Progress in Physical Geography 31: 213–225.
- Gibson, R. H., I. L. Nelson, G. W. Hopkins, B. J. Hamlett, and J. Memmott. 2006. “Pollinator webs, plant communities and the conservation of rare plants: Arable weeds as a case study.” Journal of Applied Ecology 43: 246–257.
- Guerrero, I., C. P. Carmona, M. B. Morales, J. J. Oñate, and B. Peco. 2014. “Non-linear responses of functional diversity and redundancy to agricultural intensification at the field scale in Mediterranean arable plant communities.” Agriculture, Ecosystems & Environment 195: 36–43.
- Gunton, R. M., S. Petit, and S. Gaba. 2011. “Functional traits relating arable weed communities to crop characteristics.” Journal of Vegetation Science 22: 541–550.
- Henckel, L., L. Börger, H. Meiss, S. Gaba, and V. Bretagnolle. 2015. “Organic fields sustain weed metacommunity dynamics in farmland landscapes.” Proceedings of the Royal Society B 282: 20150002.
- Hodgson, J., J. P. Grime, R. Hunt, and K. Thompson. 1995. The electronic comparative plant ecology. London: Chapman and Hall.
- Hooper, D. U., F. S. Chapin, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. “Effects of biodiversity on ecosystem functioning: a consensus of current knowledge.” Ecological Monographs 75 (1): 3–35.
- Jauzein, P. 1995. Flore des champs cultivés. Paris: Sopra-INRA.
- Julve, P., 1998. Baseflor. Index botanique, écologique et chorologique de la flore de France. http://perso.wanadoo.fr/philippe.julve/catminat.htm
- Knop, E. 2016. “Biotic homogenization of three insect groups due to urbanization.” Global Change Biology 22 (1): 228–236.
- Laliberté, E., P. Legendre, and B. Shipley. (2014). “FD: measuring functional diversity from multiple traits, and other tools for functional ecology.” R package version 1.0-12. https://CRAN.R-project.org/package=FD
- Lavorel, S., and E. Garnier. 2002. “Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail.” Functional Ecology 16 (5): 545–556.
- Le, S., J. Josse, and F. Husson. 2008. “FactoMineR: An R Package for Multivariate Analysis.” Journal of Statistical Software 25 (1): 1–18.
- Legendre, P. 2014. “Interpreting the replacement and richness difference components of beta diversity.” Global Ecology and Biogeography 23 (11): 1324–1334. doi:10.1111/geb.12207.
- Lockwood, J. L., T. M. Brooks, and M. L. McKinney. 2000. “Taxonomic homogenization of the global avifauna.” Animal Conservation 3: 27–35.
- MacKinney, M. L., and J. L. Lockwood. 1999. “Biotic homogenization: a few winners replacing many losers in the next mass extinction.” Trends in Ecology and Evolution 14: 450–453.
- Mamarot, J. 2002. Mauvaises herbes des cultures. 2nd ed. Paris: ACTA.
- Marshall, E. J. P., V. K. Brown, N. D. Boatman, P. J. W. Lutman, G. R. Squire, and L. K. Ward. 2003. “The role of weeds in supporting biological diversity within crop fields.” Weed Research 43 (2): 77–89.
- Mason, N. W. H., D. Mouillot, W. G. Lee, and J. B. Wilson. 2005. “Functional richness, functional evenness and functional divergence: the primary components of functional diversity.” Oikos 111: 112–118.
- Mouchet, M. A., S. Villéger, N. W. H. Mason, and D. Mouillot. 2010. “Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules.” Functional Ecology 24: 867–876.
- Murphy, C. E., and D. Lemerle. 2006. “Continuous cropping systems and weed selection.” Euphytica 148: 61–73.
- Nicholls, C. I., and M. A. Altieri. 2013. “Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review.” Agronomy for Sustainable development 33 (2): 257–274.
- Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. M. Stevens, E. Szoecs, and H. Wagner 2016. “vegan: Community Ecology Package.” R package version 2.4-0. https://CRAN.R-project.org/package=vegan
- Olden, J. D., and N. L. Poff. 2003. “Toward a mechanistic understanding and prediction of biotic homogenization.” American. Naturalist 162: 442–460.
- Olden, J. D., and N. L. Poff. 2004. “Ecological mechanisms driving biotic homogenization: testing a mechanistic model using fish faunas.” Ecology 85: 1867–1875.
- Olden, J. D., and T. P. Rooney. 2006. “On defining and quantifying biotic homogenization.” Global Ecology and Biogeography 15: 113–120.
- Perronne, R., V. Le Corre, V. Bretagnolle, and S. Gaba. 2015. “Stochastic processes and crop types shape weed community assembly in arable fields.” Journal of Vegetation Science 26 (2): 348–359.
- Petchey, O. L., and K. J. Gaston. 2002. “Extinction and the loss of functional diversity.” Proceedings of the Royal Society B: Biological Sciences 269 (1501): 1721–1727.
- Pinke, G., and R. M. Gunton. 2014. “Refining rare weed trait syndromes along arable intensification gradients.” Journal of Vegetation Science 25: 978–989.
- Pocock, M. J. O., D. M. Evans, and J. Memmott. 2012. “The robustness and restoration of a network of ecological networks.” Science 335 (6071): 973–977.
- Podani, J., and D. Schmera. 2011. “A new conceptual and methodological framework for exploring and explaining pattern in presence - absence data.” Oikos 120 (11): 1625–1638.
- Pointereau, P., M. L. Paracchini, J.-M. Terres, F. Jiguet, Y. Bas, and K. Biala. 2007. Identification of High Nature Value Farmland in France Through Statistical Information and Farm Practice Surveys. Luxembourg: Office for Official Publications of the European Communities.
- Rahel, F. J. 2000. “Homogenization of fish faunas across the United States.” Science 288: 854–856.
- R Core Team. 2015. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Requier, F., J. F. Odoux, T. Tamic, N. Moreau, M. Henry, A. Decourtye, and V. Bretagnolle. 2015. “Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds.” Ecological Applications 25: 881–890.
- Rollin, O., G. Benelli, S. Benvenuti, A. Decourtye, S. D. Wratten, A. Canale, and N. Desneux. 2016. “Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture.” A review. Agronomy for Sustainable Development 36.
- Richner, N., R. Holderegger, H. P. Linder, and T. Walter. 2015. “Reviewing change in the arable flora of Europe: A meta-analysis.” Weed Research 55 (1): 1–13.
- Rosenfeld, J. S. 2002. “Functional redundancy in ecology and conservation.” Oikos 98 (1): 156–162.
- Rooney, T. P., S. M. Wiegmann, D. A. Rogers, and D. M. Waller. 2004. “Biotic impoverishment and homogenization in unfragmented forest understory communities.” Conservation Biology 18: 787–798.
- Scott, M. C. 2006. “Winners and losers among stream fishes in relation to land use legacies and urban development in the southeastern US.” Biological Conservation 127: 301–309.
- Smart, S. M., K. Thompson, R. H. Marrs, M. G. Le Duc, L. C. Maskell, and L. G. Firbank. 2006. “Biotic homogenization and changes in species diversity across human-modified ecosystems.” Proceedings of The Royal Society B-Biological Sciences 273: 2659–2665.
- Tilman, D., K. G. Cassman, P. A. Matson, R. Naylor, and S. Polasky. 2002. “Agricultural sustainability and intensive production practices.” Nature 418 (6898): 671–677.
- Tison, J.-M., and B. de Foucault. 2014. Flora Gallica. Flore de France. Mèze: Biotope.
- Van der Pijl, L., 1982. Principles of dispersal in higher plants. Berlin: Springer Eds.
- Vellend, M., K. Verheyen, K. M. Flinn, H. Jacquemyn, A. Kolb, H. van Calster, G. Peterken, B. J. Graae, J. Bellemare, O. Honnay, J. Brunet, M. Wulf, F. Gerhardt, and M. Hermy. 2007. “Homogenization of forest plant communities and weakening of species-environment relationships via agricultural land use.” Journal of Ecology 95: 565–573.
- Vitousek, P. M., H. A. Mooney, J. Lubchenco, and J. M. Melillo. 1997. “Human Domination of Earth's Ecosystems.” Science 277: 494–499.
- Wezel, A., S. Bellon, T. Doré, C. Francis, D. Vallod, and C. David. 2009. “Agroecology as a science, a movement and a practice. A review.” Agronomy for sustainable development 29 (4): 503–515.
- Wisler, G. C., and R. F. Norris. 2005. “Interactions between weeds and cultivated plants as related to management of plant pathogens.” Weed Science 53 (6): 914–917.
