Abstract
Nitellopsis obtusa usually inhabits deep lakes, where the plants display vegetative growth and survive through the winter. In the present study we investigated a shallow lake where Nitellopsis reproduces sexually; we were interested in the effect of light conditions, temperature and water level on sexual reproduction of the species and its ability to survive lakebed drying. We estimated the distribution of all macrophyte species each July from 2009 to 2014 and correlated annual variations with fluctuations in hydrological conditions. We also sampled a variety of morphological characteristics of N. obtusa plants in nine periods throughout the 2009 and 2010 growth seasons and related them to water depth, day length and accumulated heat energy (growing degree-day) at the date of sampling. N. obtusa population was negatively impacted by decrease of water level, probably through the combined effects of competition with fast-growing pioneer species and lack of intrinsic ecological requirements. The fertility of N. obtusa could be interpreted as a response to disturbance in external conditions, leading to the production of long-lived and drought-resistant oospores. Development of male shoots prior to female shoots and their persistence throughout the season probably optimize the fertilization process. Sexual reproduction seems to be controlled by light and temperature conditions. An alternative hypothesis suggests that fertile and sterile populations belong to two distinct ecotypes.
Introduction
Nitellopsis obtusa (Desv.) J.Groves, 1919 is a dioecious characean species, with a patchy native distribution throughout Europe and Asia. Plants grow tall (from 20 to 120 cm) and are generally found in depths ranging from 2–14 m in permanent lakes and in still waters of secondary river channels (Corillion Citation1975; Korsch, Raabe, and van de Weyer Citation2008; Rey-Boissezon and Auderset Joye Citation2015).
Several expansions of the native geographical distribution of N. obtusa were reported during the last three decades in the Northern hemisphere (Auderset Joye and Schwarzer Citation2012; Bailly and Schaefer Citation2010; Gabka Citation2009; Korsch, Raabe, and van de Weyer Citation2008; Krause Citation1985). These changes seem to coincide with the recent acceleration of climate warming (Masson-Delmotte et al. Citation2013). In addition, N. obtusa was reported to appear in non-native North-Eastern America in the 1970s. It is spreading very rapidly throughout the Great Lakes basin and is considered invasive (Geis et al. Citation1981; Midwood et al. Citation2016; Schloesser and Manny Citation1986; Sleith et al. Citation2015). In their recent study into the realized niches of N. obtusa, Escobar et al. (Citation2016) predicted that future changes in temperature and precipitation could have a large influence on the native and invasive distributions of N. obtusa.
An expansion of the native distribution area of N. obtusa in Switzerland is also expected with the rise of mean July aerial temperatures and an increase in the size of water bodies (Auderset Joye and Rey-Boissezon Citation2015). In Switzerland, N. obtusa is colonizing lowland lakes with mesotrophic and moderately to highly mineralized waters, i.e. large water bodies with a strongly urbanized drainage basin (Rey-Boissezon and Auderset Joye Citation2015). In agreement with a study conducted in North America (Midwood et al. Citation2016), elevated conductivity and nutrient content (as indicators of human disturbance) promote the establishment of N. obtusa. However, there are still gaps in our knowledge that need to be addressed to predict the future distribution and responses of the species more precisely, in particular its phenology and its interactions with other aquatic plants.
In this paper, we describe a population of N. obtusa with remarkably high fertility due to the presence of both male and female plants, which we discovered in a shallow gravel pit lake, Bois d’Avaz, in the French Alps. The female plants produce calcified oospores (i.e. gyrogonites). We monitored water levels, temperature fluctuations and distribution of macrophyte species (percentage cover) in this shallow lake over six years (2009–2014). Significant annual and longer-term variations of water regime were observed before and during our study, leading to unpredictable wet/dry cycles (see Rey-Boissezon and Auderset Joye Citation2012 and hereafter). The abundance of N. obtusa and co-occurring species varied greatly during the six years of our monitoring. The analysis of the life cycle of N. obtusa in relation to hydrological conditions, temperature and light availability, as well as relationships to the other aquatic plant species, will increase our understanding of this Characeae’s sensitivity to a drier and warmer climate. We are assuming that the observed changes are a response to water level fluctuations and to the variations in interspecific relationships. Indeed, the dry phases may decrease the abundance of N. obtusa by destroying its above-ground biomass and, indirectly, by the increasing abundance of stress-tolerant species (Connell and Slatyer Citation1977).
We estimated the abundance of macrophyte species each July from 2009 to 2014 and explored their response to variations in hydrological conditions. We also measured a variety of morphological characteristics of N. obtusa plants, sampled every two or four weeks throughout the 2009 and 2010 growth seasons (years of high abundance for this species) and related it to water depth, day length and accumulated heat energy at the sampling date.
Materials and Methods
Study site
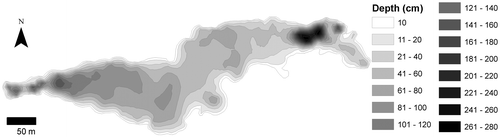
Lake Bois d’Avaz is situated in the French pre-Alps (Haute-Savoie) in the intermediate basin of the Arve River (46.070°N / 6.445° E) at an altitude of 452 m. Its surface area is approximately 47’000 m2 and its perimeter 1600 m. It is a former gravel pit created in the 1980s that was partially filled by silt originating from an exploited gravel pit. The site is shallow, characterized by a depth below 1 m for 75% of its surface area during our six-year survey. The deepest parts are small and located at the western (up to 2 m) and the eastern (up to 3 m) sides of the lake (Figure ). It receives water from rain, a hillslope aquifer and a small temporary tributary. According to our previous study (Rey-Boissezon and Auderset Joye Citation2012), the chemical composition of water showed important variations between 2009 and 2011. Globally, Lake Bois d’Avaz has oligo to mesotrophic waters, clear to slightly turbid and strongly calcareous (Table ).
Table 1. Physical and chemical characteristics of the water of Lake Bois d’Avaz based on 11 sampling dates between October 2009 and October 2011. n = total number of analyses (see Rey-Boissezon and Auderset Joye Citation2012 for details).
Water level
Water level was measured every 4 h by a data logger, from March 2009 to February 2011 (Rey-Boissezon and Auderset Joye Citation2012). The daily water levels from a previous period (since the beginning of 2004) and for February 2011 to 2014 were added from a Generalized Boosted Regression Model (G.B.M.) using hydrological and climatic data inputs from an adjacent piezometer and four meteorological stations. The model was calibrated with 70% of the randomly selected data. The 30% remaining was used as a test data-set. We employed a 10-fold cross-validation to evaluate the predictive power of our model. The package “caret” (Kuhn Citation2013) on R software (R Core Team Citation2016) was used to implement this G.B.M.
As we assume the periods of lakebed air exposure and their duration to be more critical for the aquatic vegetation than the water level variations, these were calculated for each year using the quartiles of the lake depth. Using River Analysis Package software (© eWater C.R.C. 2012), duration of drought of more than 50% of the lake (“L50”) was calculated by counting the number of days with water level at and below the median depth of the lake (-0.68 m). Extreme low water level period (“L75”) was defined as a period during which more than 75% of the area was dry, hence corresponding to area at and below 1 m deep.
Community changes over time
Macrophyte species abundance was visually estimated as percentage cover. The depth was measured each mid-July from 2009 to 2014 in 0.25 m2 square plots. These were positioned 5–10 m apart with transects spaced along 20 m perpendicular to the longest shore (Angélibert et al. Citation2010). The number of quadrats varied between 120 (2013) and 263 (2012), as a function of the area submerged.
We grouped submerged and anchored macrophyte species in four phytosociological alliances, following the reference for Switzerland (Table ) (Prunier et al. Citation2017). Each group collated species of similar growth form and ecology. N. obtusa, which normally belongs to Charion globularis, was considered separately to compare the variation in its abundance with that of each alliance.
Table 2. Phytosociological units of submerged aquatic plants according to the Swiss reference (Prunier et al., Citation2017).
To identify changes in the structure of the community over time, we performed Indicator Species Analysis (“Indval”) (Dufrêne and Legendre Citation1997). An indicator value of 1 (100%) for a particular date means that the considered species or alliance is present (i) only at that date (maximal specificity) and (ii) in all quadrats on this date (maximal fidelity). Statistical significance of each indicator value was checked with a permutation test (1000 permutations). We ran the analysis using the labdsv package (Roberts Citation2016).
Nitellopsis obtusa life cycle
To describe N. obtusa phenology, we collected samples every two to four weeks from March to December 2009 and from March to October 2010 (13 and 14 sampling dates, respectively). In order to collect a maximal amount of plants, samples were spatially allocated according to the distribution of the species on the day of the visit. Consequently, sampling was not random, because of the unpredictable spatial and temporal variability of N. obtusa occurrence. When possible, plants were sampled from a maximum of 10 locations per date to highlight spatial heterogeneity of growth. However, since N. obtusa population regressed from 2009 to 2011 (see results section), the number of samples differed between 2009 and 2010 (65 and 45 sampling locations, respectively). Plant samples were carefully collected by hand, or using a heavy hook, to obtain the entire plant. The origin of each plant from oospore germination or vegetative propagules (bulbils or thallus fragments) was determined. N. obtusa samples were fixed in 70% ethanol and rehydrated with tap water before biometric measurements. The laboratory observations and biometric measurement were made using a Leica stereomicroscope M205C with a maximum magnification of 160x. To assess the plant phenology, the following developmental stages were described: new sprouts, sterile plant, proportion of ripe gametangia (antheridia – male, oogonia – female or oospore – the product of gametes fertilization), the proportion of abortive oogonia (failure of fertilization process), calcification of oospores, signs of decay. Height, axis diameter, internode length, branchlet length, antheridia diameter, oospore and gyrogonite (calcified oospore) morphology (length, width, spiral number, spiral width, apical and basal morphology) were recorded. Depending on the phenological stage of the plants (early or late stage of fructification, decay), the number of male or female gametangia was measured to calculate minimal, maximal and mean values of variation among individuals (from 4 to 31 for antheridia, from 6 to 56 for gyrogonites).
The description of species life cycle was based on the accumulation of heat units over time instead of time only, known as “growing degree-days” (G.D.D.) (Spencer et al. Citation2000; Zalom et al. Citation1983). The calculation of G.D.D. requires the identification of a low temperature threshold at and below which the growth of the species is null. This limit is not well defined for stoneworts (Boissezon Citation2014). In the absence of accurate data, we set 4 °C as the lowest temperature threshold. This was the minimal winter temperature recorded in our study site and more generally in temperate ponds (Rey-Boissezon and Auderset Joye Citation2012). The calculation of G.D.D. also requires the definition of a starting date, known as biofix, corresponding to a biological event. In this study we decided to set the biofix at the 16th of March, two weeks before the observation of the first new shoots. Using the water temperature recorded by loggers, we calculated G.D.D. for 2009 and 2010 with the “actual temperature method”. This method uses the logging interval of the temperature data (here 4 h) to perform a numerical integration. The area between the curve and the low threshold is then used to compute G.D.D..
The G.D.D. are accumulated through the year, whereas changes in water level and in day length follow seasonal trends. The day length is astronomically defined and varies in the same way each year for a given latitude. To explore the onsets of phenological events over time, we grouped Nitellopsis samples into periods of one to two weeks with characteristics described in Table (results section). The 110 plant samples were characterized by several biological attributes consisting of a mixture of continuous and qualitative descriptors. In this study, we were particularly interested in understanding the environmental conditions under which N. obtusa reproduced sexually. We calculated the relative frequency of young sterile, male, female and senescent plants at each sampling period.
All statistical analyses were performed using ade4 (Dray and Dufour Citation2007) and labdsv (Roberts Citation2016) in R free software (R Core Team Citation2016).
Results
Water level fluctuations
Two low water level periods occurred between 2004 and 2014: from 2004 to the beginning of 2006 and from 2009 to 2011 (Figure , supplemental material 1). Water levels decreased during summer and autumn 2004 to 2005: 50% (L50) of the lake area dried during 73 and 87 days, respectively. The drought over 75% of the lake area lasted only one week during autumn 2005. It took approximately 50 days at the beginning of 2006 to flood half of the lake area again. During 2006, 2007 and 2008, most of the total surface area remained inundated. In summer 2009 and 2010 water levels decreased substantially, leading to the progressive drying out of more than 75% of the lake area at the end of autumn (respectively 133 and 198 days below L50, 61 and 33 days below L75). Low regional rainfall during the 2010–2011 winter prevented the complete re-filling of the groundwater table. This low water level phase continued until October 2011. The three following years, i.e. 2012 to 2014, were characterized by a return to high water levels.
Figure 2. Annual duration of low level phases. L50 = low water level at which 50% of the surface area dried out (−0.68 m). L75 = low water level at which 75% of the surface area dried out (−1 m).
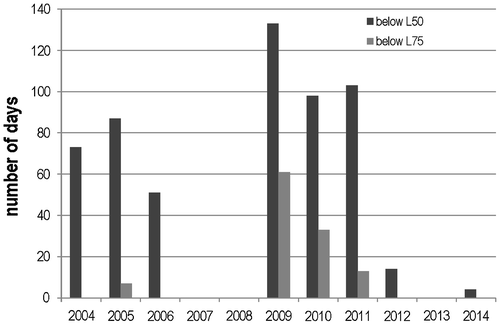
Consequently, the shallower parts of lake Bois d’Avaz were subjected to wet/dry annual and seasonal phases for 10 years, whereas the deepest parts (>130 cm) remained inundated.
Changes in aquatic plant community
Field records showed annual variations in the distribution of N. obtusa (Figures ). In summer 2009, N. obtusa was dominating the plant community. In 2010 and 2011, the frequency of the species decreased and almost disappeared in 2011, except in the deepest small area in the eastern side of the lake (Figures ). A progressive recolonization was observed from 2012. However, by comparison with 2009, the N. obtusa population still had not recovered completely in summer 2014.
Figure 3. Spatial and temporal variation in the abundance of N. obtusa. The scale is expressed as the percentage of cover in each quadrat. Small points represent the quadrats without N. obtusa.
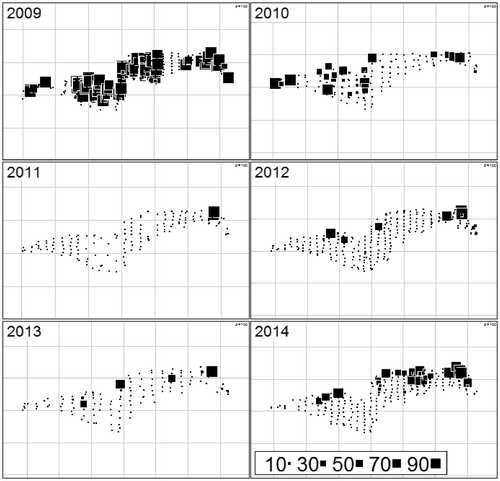
Figure 4. Indval value of N. obtusa and four alliances at each summer. All indval values are statistically significant (p value < 0.001).
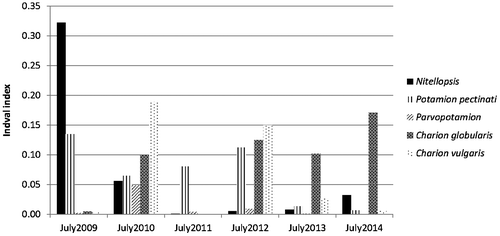
The 4 phytosociological alliances varied over time in a different way than N. obtusa (Figure & supplemental materials 2 to 5). In 2009 only species of the Potamion pectinati co-occurred with N. obtusa. The following summer, the submerged plant community consisted of a mixture of all phytosociological units and N. obtusa decreased. The populations of species composing the Charion vulgaris exploded during this year (supplemental material 5). In 2011 the community was essentially characterized by species of the Potamion pectinati again (supplemental material 2). This latter alliance was more or less stable between 2009 and 2012, while in 2013 and 2014 it was not dominant (very low indval value). In summer 2012 we observed a well-mixed community with the return of Charion vulgaris and other species belonging to Charion globularis. This latter alliance finally dominated the community of submerged plants for the last two years of our study, i.e. 2013 and 2014 (supplemental material 4).
Morphology and life cycle
Morphological observations and biometric measurements provided an accurate description of N. obtusa growing in the study sites (Figure , Table ). The average height was around 29 cm, with maximum of 100 cm (Table ). In most cases, shoots were dark green, with brownish upper parts in shallow waters (<1 m deep). Lower axial nodes produced large white stellate bulbills (Figure f). Gametangia were formed on all branchlet nodes (Figure a, b), solitary or geminate, so that each fructified plant could theoretically produce up to 60 gametangia. Unripe antheridia were orange-coloured and less than 800 μm in diameter. Ripe antheridia were bright red in colour and 800–1500 μm in diameter (Figure b, Table ). Oogonia were nearly spherical, coloured from bright red (shallow waters) to light green before fertilization and ripening of the zygote (oospore). Oogonia exhibited a very small five-celled coronula. Non-calcified oospores were ellipsoidal with a truncated basis, of russet-brown to dark-brown colouring (wet or dry oospores). These were rare, as calcification took place immediately after ripening. Gyrogonites varied from inverted-pear to sub-cylindrical shapes (Figure c). Spiral cells were concave to smooth, sometimes slightly convex. Spiral index (gyrogonite length/spiral width) was low at around 6. Each gyrogonite showed an apical peripheral groove and prominent apical nodes (Figure d). The base was broadly truncated in the lateral view, due to a bowl-shaped depression surrounding the basal plug (Figure e). Gyrogonites were voluminous, with dimensions around 1070 μm in length and 890 μm in width, but exhibited large variation in size over time, as intracellular biomineralization is a progressive process (Soulié-Märsche Citation1979). The isopolarity index (ISI = 100*length/width) was relatively constant, around 120 (Table ).
Figure 5. N. obtusa from the lake Bois d’Avaz. (a) Female specimen with ripe calcified oospores (22 September 2010, ≈ 2700 G.D.D.). (b) Male specimen with ripe antheridia (27 July 2010, ≈ 1850 G.D.D.). (c–e) Lateral, apical and basal view of a gyrogonite. (f) Star-shaped bulbil. (photos: Boissezon A).
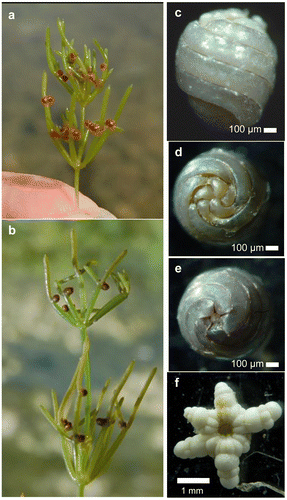
Table 3. Morphological and sexual features of N. obtusa from lake Bois d’Avaz.
In years 2009 and 2010, all observed new shoots were clonal specimens, grown from bulbils (on rhizoids) or nodes of thallus fragments. Indeed, oospore germination failed to be observed in spite of the presence of many gyrogonites in the sediment in spring of both years.
For dioecious species, such as N. obtusa, the fertilization of oogonia by spermatozoids requires that male plants bearing ripe antheridia and female plants occur at the same time, in the same space or in distinct patches separated by short distances. Otherwise, oogonia cannot be fertilized, and thus abort. Amongst the 110 samples collected in different parts of the lake throughout 2009 and 2010 growth seasons, we found 35 samples in which male and female plants were mixed (32%). Further, 28 samples consisted of female plants only and 14 of male plants only (31 and 16% respectively); 33 remaining samples were sterile (30%).
To explore the onsets of phenological events over time (sterile, fructified male or female plants, senescent plants) we grouped N. obtusa samples into periods of one to two weeks (when possible), with characteristics described in Table . A more detailed analysis of the variation in proportions of ripe gametangia and abortive oogons developed by fructified plants throughout those periods is given in Figure .
Table 4. Characteristics of sampling periods and relative frequency (%) of phenological phases of N. obtusa (maximal value for each phenophase is in bold).
Figure 6. Proportion of (a) ripe antheridia, (b) oospores and (c) abortive oogonia observed on N. obtusa at different periods. See Table for more details about the sampling periods. P-values from Kruskal–Wallis comparison tests are all significant.
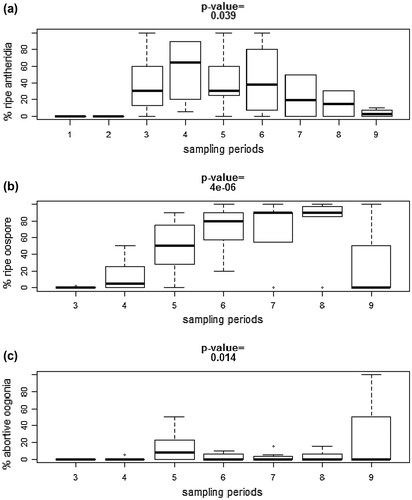
The frequency of sterile, male, female and senescent plants in the population varied as the season progressed (Table ). From March to May (<700 G.D.D. and between 13 and 15 h of day length, maximal water level), all new shoots were sterile or male. More energy was necessary to induce the appearance of the first female shoots, which were collected 400 G.D.D. after the first antheridia and during the longest days (16 h). By then the water level had already decreased by approximately 28 cm. The ripening of antheridia over time did not follow the same time course as the ripening of oospores (Figure A, B). Antheridia ripening showed a bell-shaped pattern, reaching its maximum during the first two weeks of July, i.e. between 1200 and 1600 G.D.D., and during the longest days (16 h). For the rest of the season, the proportion of ripe antheridia decreased progressively (Figure A), while the frequency of collected male plants stayed relatively constant throughout the season (Table ). Consequently, after mid-July and until the end of the growing season new male specimens with unripe antheridia occurred continuously. By contrast, the proportion of ripe oospores increased progressively until the end of the growing season (Figure B). Nevertheless, the production of spermatozoids at the very end of the growth season appeared to be insufficient to fertilize females, as indicated by the drastic increase of abortive oogonia proportion up to 2900 G.D.D. and daylight decreasing to 11 h (Figure C).
Discussion
In the present study, no other environmental variable was measured in addition to the water temperature, the day length and the water level. We assume that these variables are sufficient at a single site, where most environmental variables are likely be correlated with topography, frequency and duration of flooding/lakebed drying. By gathering species in life-strategy, phytosociological units, we integrated community effects (competition) and life-history traits as additional factors to explain the observed variations. This is clearly relevant in this context, as one of the main impacts of lakebed air exposure on aquatic plant communities is to destroy non-tolerant species, thus reducing competition intensity.
Lakebed drying-sensitivity
Drying of the lakebed is recognized as a disturbance that macrophyte communities must accommodate (Bunn and Arthington Citation2002; Davies and Walker Citation1986). Lakebed air exposure controls the composition of aquatic plant communities by eliminating standing competitors and so creating gap opportunities for the recruitment of fast-growing pioneer species (Connell and Slatyer Citation1977). Lake Bois d’Avaz can be considered a semi-permanent water body, since major water-level drawdowns occur only during the warmest and driest years. These random fluctuations allowed us to observe the response of aquatic plants to a wide range of inundation conditions and justified our thorough sampling effort. As expected, we observed spatial and temporal dynamics of the macrophyte assemblage in parallel to annual variations in water level. The availability of meteorological and groundwater-level data enabled modelling of the hydrological regime of the lake from 2004 (supplemental material 1). The large mats of N. obtusa and other species from the Potamion pectinati (Potamogeton pectinatus L., Myriophyllum spicatum L., Najas marina L.) observed in July 2009 could be the result of five consecutive years (2004–2008) without extreme low-water level events, rather than just the conditions in 2008. Between 2010 and 2014 N. obtusa was replaced by other macrophyte species in the parts of the lake that were exposed to the air (<1.30 m), while it remained dominant and survived through winter in a deepest small area. In 2010 and 2012, i.e. years succeeding an important water-level drawdown, the community was enriched with fast-growing pioneer species from the Charion vulgaris alliance, in particular Tolypella glomerata (Desv.) Leonh. and Chara aspera Willd. (Boissezon Citation2014). From 2012 to 2014, the progressive recolonization of the lake by species characterizing the Charion globularis alliance (e.g. Chara contraria Kütz., C. hispida L.) can be interpreted as a positive response to three succeeding years of high water level. Consequently, the low recovery of N. obtusa is probably due, at least partially, to the competition with species that are better adapted to lakebed air exposure-disturbance.
This apparent lakebed drying-sensitivity of N. obtusa could explain its current distribution mainly in large and deep permanent water bodies. However, despite the obvious negative impact of drawdowns on N. obtusa, it is still impossible to deconstruct the direct effects related to intrinsic ecological requirements and the indirect effects related to competition with the more pioneer species described previously (C. hispida and C. contraria). Indeed, the germination and establishment of stonewort species under particular environmental conditions is also related to their life history, i.e. longevity (annual or perennial), breeding systems (monoecious or dioecious), timing and trigger of germination of propagules, growth rates and reproductive type (sexual or asexual), and capacities for morphological and reproductive adaptations to environmental changes – i.e. plasticity (Casanova Citation1994; Casanova and Brock Citation1999a). The phenology of N. obtusa analysed here brought new key understanding of its hydrological preferences and adaptive abilities.
Reproductive mode
N. obtusa is a perennial species that forms dense and large mats in stagnant or slow-running freshwaters. In such conditions it survives vegetatively by means of bulbils and overwintering of the above-ground parts, while rarely producing antheridia and, even more rarely, oogonia (Corillion Citation1975; Langangen Citation2007; Migula Citation1897; Stewart and Church Citation1992). Thus, the occurrence of fertile N. obtusa and co-occurrence of male and female plants in approximately one third of the samples in lake Bois d’Avaz is significant.
During 2009 and 2010, N. obtusa required continuous inundation for at least seven months to build a dense oospore bank and did not survive winter, except in the deepest eastern part of the lake. This slow growth might partially explain why the species needs permanent waters to develop. Indeed, there are two ways to overcome drought disturbance during the season of growth: (i) find refuge in deep permanently flooded areas (perennial or annual species), (ii) establish in areas that dry out only at the end of the growing season and produce resistant dormant propagules before the onset of the unfavourable period (necessary for annual species). Bociąg and Rekowska (Citation2012) proved that clonal life strategy is used by some Chara species living in deep permanent conditions. Vegetative growth is possible through the development of new shoots from rhizoidal bulbils or from axial nodes. In the present study, N. obtusa exhibited both sexual and clonal reproduction, as bulbils were systematically present on N. obtusa rhizoids and stems. However, clonality alone may be less successful in temporary habitats, where the lifespan of thallus fragments is limited by winter freezing or summer drying.
According to several studies, recently Nitellopsis has appeared to abandon the deepest parts of lakes to colonize shallower waters, where it can completely fill the water column and produce gametangia (Richard Lansdown, personal comm., own observations). Previously, Krause (Citation1985) reported a fertile population producing large amounts of ripe antheridia and oospores. Similarly to the present study, he found this population in shallow waters (0.5–1.5 m) of a gravel-pit lake at Altenheim (southern Germany). The fertility of N. obtusa in lake Bois d’Avaz could thus be interpreted as a response to disturbed conditions, ensuring that long-lived and drought-resistant oospores are produced in order to guarantee the species’ survival (Boissezon Citation2014). Indeed, oospores, and particularly gyrogonites, found in the sediment, are adapted to long-term survival in a dormant stage, unlike bulbils, which probably ensure short-term persistence of species (Van den Berg, Coops, and Simons Citation2001). However, no germination of N. obtusa gyrogonites from the seed bank were observed during our monitoring, suggesting that the environmental cues for dormancy break were not met. The persistence of gyrogonite dormancy after a water-level rise indicates that the stimuli for dormancy break are complex. This mechanism ensures a constant presence of propagules in the seed bank and a minimum loss of individuals under non-optimal conditions (Brock Citation2011; Brock et al. Citation2003). The difficulty for N. obtusa to re-establish after successive droughts, i.e. its low resilience, could be caused partially by the absence of required stimuli for the germination of the seed bank. Further research is needed to understand the role of oospores bank for N. obtusa resilience and dispersion.
We demonstrated that the ratio of male and female specimens changed as the season progressed (i.e. as heat accumulated), the water level decreased (which induces an increase of the underwater luminosity) and the day length changed. The ripening of antheridia required less energy in terms of heat and light than did oogonia ripening. New male specimens occurred throughout the season. The development of male organs before female ones, known as “protandry”, is a common phenomenon in stoneworts (Casanova Citation1994; Casanova and Brock Citation1999b; Guerlesquin Citation1987). In the Paarsteiner lake (Germany), Migula (Citation1897) found N. obtusa bearing few ripe antheridia in mid-September, and only one specimen with two ripe oospores two months later. According to Cox (Citation1981), this observation could be interpreted as a temporal niche partitioning between sexes. Based upon G.D.D. and day duration, another explanation could be that antheridia are less expensive to produce than oogonia, in which starch and lipids are stored. The lower cost of antheridia production may allow male plants to develop continuously during the whole growth season, as was observed in the lake Bois d’Avaz. Thus, spermatozoids are permanently available, optimizing the fertilization process.
Phenological plasticity or ecotype?
Fossil gyrogonites of N. obtusa were observed in quaternary sediments of Europe and Asia, suggesting that the species used to reproduce sexually during this period. They have also been found in Sahara within Early Holocene deposits, i.e. 4500 y B.P., where living populations are no longer present (Soulié-Märsche et al. Citation2002). Despite the gap in our knowledge about the factors influencing N. obtusa production of gyrogonites, those fossil remains were interpreted as a sign of “cold”, relatively deep (4–12 m) and permanent conditions (Kropelin and Soulié-Märsche Citation1991; Soulié-Märsche Citation1993) by analogy with the preferential habitat of current populations. Consequently, the present study provides new results that can be applied in palaeolimnological studies. One assumption is that N. obtusa has more adaptive abilities than suggested by its current distribution in deep and permanent lakes of the Northern hemisphere. The deepest part of this habitat could be sub-optimal in terms of light and/or temperature to favour the development of oogonia, and could explain why only sterile or male individuals are generally observed. N. obtusa probably grows in those sub-optimal conditions because of the competition with other aquatic plants in shallower waters. An alternate hypothesis suggests that fertile and sterile populations belong to two distinct ecotypes of shallow and deep habitats, respectively.
On a regional scale, the expansion of N. obtusa seems to be contemporary with the progressive eutrophication of European lakes and the acceleration of climate warming during the last three decades. The long fossil history of N. obtusa, complemented by the results provided by the present study, suggests that current N. obtusa, or its shallow habitat ecotype, might be “hanging on” in Europe as a remnant of a formerly wider distribution, waiting for conditions to become more suitable for expansion and sexual reproduction. Further research is needed to understand how the reproductive mode of N. obtusa is genetically and environmentally determined.
In conclusion, comparative long-term studies are needed to define with accuracy the interactive influences of light and temperature on N. obtusa phenology. For this purpose, populations growing in shallow waters, located at high latitudes, and from deep parts of lakes could be monitored together with light and temperature and water-level fluctuations. Results from fieldwork would need to be complemented by growth experiments in laboratory with controlled light, temperature and water-level conditions. In addition, the study of the viability and dormancy break signal of N. obtusa oospores and bulbils could be important to define the dispersal and resilience capacity of N. obtusa populations. Finally, genetic analyses of male and female individuals are required to distinguish high phenological plasticity from ecotypes. Such research could help in predicting (i) native N. obtusa distribution under warmer and drier climatic conditions, (ii) its invasive risk in recently colonized countries and (iii) its potential to be used as a bio-indicator of climate change impact on freshwaters systems.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
A. Boissezon is employed as a scientific associate (2013–2016 at F.-A. Forel Institute and Institute of Environmental Sciences, and since 2015 at the H.E.S.-S.O. University of Applied Sciences and Arts Western Switzerland). She has been conducting research for 10 years on the ecology and phenology of the characeae of Switzerland and elsewhere, and has published her results in national and international peer-reviewed scientific journals.
Contribution: study design, data acquisition and analysis, interpretation, writing of the article.
D. Auderset Joye was employed as a scientific associate at F.-A. Forel Institute and Institute of Environmental Sciences (retired since 2016). She authored several publications in peer-reviewed scientific journals. Her research addressed the relationship between environmental factors and the biological diversity of aquatic ecosystems and distribution of macrophytes species. She is a renowned charophytologist and was co-director of the Swiss Red List.
Contribution: data acquisition, article revision.
https://figshare.com/s/478e09999c0f94e6b817
T. Garcia Under the direction of Dominique Auderset Joye and Aurélie Boissezon, Tamara Garcia obtained her master’s degree in natural environmental sciences in 2011 at the F. A. Forel Institute of the University of Geneva. The title was “Nitellopsis obtusa (Desv.) J.Groves in a shallow lake: description of the habitat and of the life cycle of the characeae” [Nitellopsis obtusa (Desv.) J.Groves dans un lac peu profond: Description de l’habitat et du cycle de vie de cette characée].
Contribution: data acquisition, laboratory analysis.
Supplementary Material
References
- Angélibert, S., V. Rosset, N. Indermuehle, and B. Oertli. 2010. “The Pond Biodiversity Index “IBEM”: A New Tool for The Rapid Assessment of Biodiversity in Ponds from Switzerland. Part 1. Index Development.” Limnetica 29 (1): 93–104.
- Auderset Joye, D., and A. Rey-Boissezon. 2015. “Will Charophyte Species Increase or Decrease Their Distribution in A Changing Climate?” Aquatic Botany 120: 73–83.10.1016/j.aquabot.2014.05.003
- Auderset Joye, D., and A. Schwarzer. 2012. Liste rouge Characées. Espèces menacées en Suisse, état 2010 [Red List of Characeae. Threatened Species in Switzerland, State 2010]. Berne: Office fédéral de l’Environnement.
- Bailly, G., and O. Schaefer. 2010. Guide illustré des Characées du Nord-Est de la France [Illustrated Guide of Characeae of Northeastern France]. Besançon: Conservatoire Botanique National de Franche-Comté.
- Bociąg, K., and E. Rekowska. 2012. “Are Stoneworts (Characeae) Clonal Plants?” Aquatic Botany 100: 25–34.10.1016/j.aquabot.2012.03.007
- Boissezon, A. 2014. “Distribution et dynamique des communautés de Characées : Impact des facteurs environnementaux régionaux et locaux” [Distribution and Dynamics of Characeae Communities: Impacts of Regional and Local Environmental Factors.] PhD-diss., Université de Genève.
- Brock, M. A. 2011. “Persistence of Seed Banks in Australian Temporary Wetlands.” Freshwater Biology 56 (7): 1312–1327.10.1111/fwb.2011.56.issue-7
- Brock, M. A., D. L. Nielsen, R. J. Shiel, J. D. Green, and J. D. Langley. 2003. “Drought and Aquatic Community Resilience: The Role of Eggs and Seeds in Sediments of Temporary Wetlands.” Freshwater Biology 48 (7): 1207–1218.10.1046/j.1365-2427.2003.01083.x
- Bunn, S., and A. Arthington. 2002. “Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity.” Environmental management 30 (4): 492–507.10.1007/s00267-002-2737-0
- Casanova, M. T. 1994. “Vegetative and Reproductive Responses of Charophytes to Water-Level Fluctuations in Permanent and Temporary Wetlands in Australia.” Australian Journal of Marine and Freshwater Research 45 (8): 1409–1419.10.1071/MF9941409
- Casanova, M. T., and M. A. Brock. 1999a. “Charophyte Occurrence, Seed Banks and Establishment in Farm Dams in New South Wales.” Australian Journal of Botany 47 (3): 437–444.10.1071/BT97099
- Casanova, M. T., and M. A. Brock. 1999b. “Life Histories of Charophytes from Permanent and Temporary Wetlands in Eastern Australia.” Australian Journal of Botany 47 (3): 383–397.10.1071/BT97086
- Connell, J., and R. Slatyer. 1977. “Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization.” American naturalist 111 (982): 1119–1144.10.1086/283241
- Corillion, R. 1975. Flore des Charophytes (Characées) du Massif Armoricain. Flore et végétation du Massif Armoricain [Flora of Charophytes (Characeae) of Massif Armoricain. Flora and Vegetation of Massif Armoricain]. Paris: Abbayes H & al.
- Cox, P. 1981. “Niche Partitioning Between Sexes of Dioecious Plants.” American Naturalist 117 (3): 295–307.10.1086/283707
- Davies, B., and K. Walker. 1986. The Ecology of River Systems. La Hague: Dr. W. Junk Publishers.10.1007/978-94-017-3290-1
- Dray, S., and A. Dufour. 2007. “The Ade4 Package: Implementing the Duality Diagram for Ecologists.” Journal of Statistical Software 22 (4): 1–20.
- Dufrêne, M., and P. Legendre. 1997. “Species Assemblages and Indicator Species: The Need for A Flexible Asymmetrical Approach.” Ecological Monographs 67 (3): 345–366.
- Escobar, L. E., H. Qiao, N. B. D. Phelps, C. K. Wagner, and D. J. Larkin. 2016. Realized Niche Shift Associated with The Eurasian Charophyte Nitellopsis obtusa becoming Invasive in North America. Scientific Reports (July): 1–11.
- Gabka, M. 2009. Charophytes of the Wielpolska Region (NW Poland): Distribution, Taxonomy and Autoecology. Poznań: Bogucki Wydawnictwo Naukowe.
- Geis, J., G. J. Schumacher, D. J. Raynal, and N. P. Hyduke. 1981. “Distribution of Nitellopsis obtusa (Charophyceae, Characeae) in the St Lawrence River - A New Record for North America.” Phycologia 20 (2): 211–214.10.2216/i0031-8884-20-2-211.1
- Guerlesquin, M. 1987. “Recherches récentes sur les Charophycées - Morphogénèse et Reproduction sexuée” [Recent Researches on Charophytes – Morphogenesis and Reproduction.] Bulletin de la Société Botanique De France-actualités Botaniques 134 (1): 17–30.10.1080/01811789.1987.10826849
- Korsch, H., U. Raabe, and K. van de Weyer. 2008. “Verbreitungskarten der Characeen Deutschlands” [Distribution Maps of Characeae of Germany.] Rostocker Meeresbiologische Beiträge: 57–108.
- Krause, W. 1985. “Über die Standortsansprüche und das Ausbreitungsverhalten der Stern-Armleuchtealge Nitellopsis obtusa (Desvaux) J.Groves” [On the Habitat Requirements and Spreading Behavior of The Starry Stonewort Nitellopsis obtusa (Desvaux) J.Groves.] Carolinea 42: 31–42.
- Kropelin, S., and I. Soulié-Märsche. 1991. “Charophyte Remains from Wadi Howar as Evidence for Deep Midholocene Fresh-Water Lakes in the Eastern Sahara of Northwest Sudan.” Quaternary Research 36 (2): 210–223.
- Kuhn, M. 2013. “Package ‘Caret’.” Accessed June 27, 2013. https://cran.r-project.org/web/packages/caret/caret.pdf
- Langangen, A. 2007. Charophytes of the Nordic Countries. Oslo: Saeculum ANS.
- Masson-Delmotte, V., M. Schulz, A. Abe-Ouchi, J. Beer, A. Ganopolski, J. F. González Rouco, E. Jansen, et al. 2013. “Information from Paleoclimate Archives.” In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by T. F. Stocker, D. Qin, G. -K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley, 383–464. Cambridge: Cambridge United Kingdom University Press.
- Midwood, J. D., A. Darwinb, Z.-Y. Hoa, D. Rokitnicki-Wojcika, and G. Grabasa. 2016. “Environmental Factors Associated with the Distribution of Non-Native Starry Stonewort (Nitellopsis obtusa) in a Lake Ontario Coastal Wetland.” Journal of Great Lakes Research 42 (2): 1–8.
- Migula, W. 1897. Die Characeen Deutschlands, Oesterreichs und der Schweiz [The Characeae of Germany, Austria and Switzerland]. Leipzig: TS. de L. Rabenhorst’s kryptogamen Flora.
- Prunier, P., F. Greulich, C. Béguin, B. Boissezon, R. Delarze, O. Hegg, F. Klötlzi, R. Pantke, P. Steiger, and P. Vittoz 2017. Un référentiel pour les associations végétales de Suisse : PhytoSuisse [A Reference for Vegetal Associations of Switzerland : Phytosuisse]. V3. https://www.infoflora.ch/fr/milieux/phytosuisse/.
- R Core Team. 2016. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Rey-Boissezon, A., and D. Auderset Joye. 2012. “A Temporary Gravel Pit as A Biodiversity Hotspot for Aquatic Plants in the Alps.” Archives des Sciences 65 (1–2): 177–190.
- Rey-Boissezon, A., and D. Auderset Joye. 2015. “Habitat Requirements of Charophytes - Evidence of Species Discrimination Through Distribution Analysis.” Aquatic Botany 120: 84–91.10.1016/j.aquabot.2014.05.007
- Roberts, D. W. 2016. “Ordination and Multivariate Analysis for Ecology.” Package “labsdv.” https://ecology.msu.montana.edu/labdsv/R.
- Schloesser, D. W., and B. A. Manny. 1986. “Distribution of Submersed Macrophytes In the St Clair-detroit River System, 1978.” Journal of Freshwater Ecology 3 (4): 537–544.10.1080/02705060.1986.9665147
- Sleith, R. S., A. J. Havens, R. A. Stewart, and K. G. Karol. 2015. “Distribution of Nitellopsis obtusa (Characeae) in New York, U.S.A.” Brittonia 67 (2): 166–172.10.1007/s12228-015-9372-6
- Soulié-Märsche, I. 1979. “Etude comparée des Gyrogonites de Charophytes actuelles et fossiles et phylogénie des genres actuels” [Comparative Study of Gyrogonites of Living and Fossil Charophytes and phylogeny of Living Genus.] PhD diss., Université des Sciences et Techniques de Montpellier 2.
- Soulié-Märsche, I. 1993. “Contribution of Fossil Charophytes to Research on Abrupt Climatic Changes.” Bulletin De La Société Géologique De France 164 (1): 123–130.
- Soulié-Märsche, I., M. Benammi, and P. Gemayel. 2002. “Biogeography of Living and Fossil Nitellopsis (Charophyta) in Relationship to New Finds from Morocco.” Journal of Biogeography 29 (12): 1703–1711.
- Spencer, D. F., G. G. Ksandera, J. D. Madsenb, and C. S. Owensc. 2000. “Emergence of Vegetative Propagules of Potamogeton nodosus, Potamogeton pectinatus, Vallisneria americana, and Hydrilla verticillata Based On Accumulated Degree-Days.” Aquatic Botany 67 (3): 237–249.10.1016/S0304-3770(00)00091-7
- Stewart, N. F., and J. M. Church. 1992. Red Data Book of Britain and Ireland: Stoneworts. Peterborough: Joint Nature Conservation Committee.
- Van den Berg, M., H. Coops, and J. Simons. 2001. “Propagule Bank Buildup of Chara aspera.” Hydrobiologia 462: 9–17.10.1023/A:1013125603555
- Zalom, F., P. Goodell, L. Wilson, W. Barnett, and W. Bently. 1983. Degree-Days: The Calculation and Use of Heat Units in Pest Management. University of California Division of Agriculture and Natural Resources Leaflet 21373. Davis, Calirfornia: Division of Agriculture and Natural Resources.