Abstract
Eichhornia crassipes is notorious as the world’s worst aquatic weed, and here we present all aspects of its biology, ecology and invasion behaviour within the framework of the new series of Botany Letters on Monographs on invasive plants in Europe. Native to the Amazon in South America, the plant has been spread around the world since the late 1800s through the ornamental plant trade due to its attractive lilac flowers, and is established on every continent except Antarctica. Its distribution is limited in Europe to the warmer southern regions by cold winter temperatures, but it has extensive ecological and socio-economic impacts where it invades. Its reproductive behaviour, characterised by rapid vegetative spread and high seed production, as well as its wide physiological tolerance, allows it to proliferate rapidly and persist in a wide range of environments. It has recently been regulated by the EU, under Regulation No. 1143/2014, which states that E. crassipes shall not be brought into the territory of the Union, kept, bred or transported to, from or within the Union. However, in the absence of effective control measures, such as herbicidal and biological control, it will continue to be a significant threat to European waterways, particularly in eutrophic waters, and under future climate change scenarios.
1. Taxonomy
1.1. Names and classification
Scientific name: Eichhornia crassipes (Mart.) Solms
Synonyms: Pontederia crassipes Mart. (Basionym), Eichhornia cordifolia Gand., Eichhornia crassicaulis Schltdl., Eichhornia speciosa Kunth, Heteranthera formosa Miq., Piaropus crassipes (Mart.) Raf., Pontederia crassicaulis Schlecht., Pontederia crassipes Roem. & Schult
Taxonomic position: Monocotyledons, Order: Commelinales, Family: Pontederiaceae
Common names: aguapé, baronesa (Brazil), jacinto-aquatico (Portugal), bisnidh, zanim, zoqqueym et.tani Baqaqa, camalote (Mexico) habba, halassandi/halassant (Egypt), buchón (Colombia), bora (Venezuela), jacinthe d’eau (France), gulbakauli (Pakistan), jacinto de agua o camalote, lechuguilla, lirio acuatico (Spain), lila de agua (Dominican Republic), tokozelka (former Czechoslovakia), top-chawa (Thailand), violeta de agua (Chile), wampee (former U.S.S.R.), Wasserhyazinthe (Germany), susümbülü (Turkey), tarulla (Colombia), vanhyacint (Denmark), water hyacinth (U.K.), waterhyacinth (U.S.A.) yakinton hamaim (Israel), jacinthe d’eau (Côte d’Ivoire), wasserhyazinthe (Namibia), wota haisin (Papua New Guinea), curse of Bengal (India), namasupuni (Malawi), putu putu (Zambia)
EPPO code: EICCR
1.2. Morphological description
1.2.1. Species description
Eichhornia crassipes is a free-floating aquatic macrophyte, which reproduces both vegetatively through daughter plant (ramet) production and sexually via seeds (Penfound and Earle Citation1948) (Figures and ). It has 6–10 shiny green leaves arranged in basal rosettes, borne on bulbous or elongate petioles, depending on crowding conditions: in dense stands, the petioles are elongate, up to 1 m tall, but in sparse infestations or at the edge of infestations, the petioles are bulbous and short (<30 cm) (Center and Spencer Citation1981). The rhizome and feathery roots are submerged, and respond to changes in nutrient availability, where longer, denser roots are associated with limited phosphorus (P) availability (Xie and Yu Citation2003).
Figure 1. Eichhornia crassipes. Drawn by W. Roux, first published in Henderson and Cilliers (Citation2002).
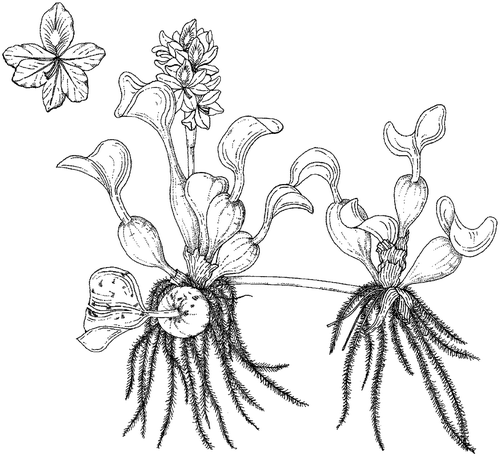
Figure 2. A flowering Eichhornia crassipes plant, illustrating the beautiful lavender-blue flowers, largely responsible for its anthropogenic spread around the world. Photograph: J. Coetzee.

The attractive flowers are pale blue to mauve and borne on inflorescences, and produce many long-lived seeds, remaining viable in seedbanks for up to 20 years (Gopal Citation1987). The flowers display the genetic polymorphism of tristyly where all flowers of an individual plant possess one of three distinct corresponding style and stamen length phenotypes (Eckenwalder and Barrett Citation1986). In its native range, the short-style forms are dominant, while in the introduced range, the intermediate-style form is prevalent, and the long-styled form less common (Barrett Citation1977; Barrett and Forno Citation1982).
1.2.2. Distinguishing features
Eichhornia crassipes is the only floating species in the genus. In its native range, E. crassipes co-occurs with the morphologically similar Eichhornia azurea (Sw.) Kunth but differs in that it is free-floating, while E. azurea is rooted. There are no other Eichhornia species outside South America, apart from E. natans (P.Beauv.) Solms which is native to tropical Africa, and readily distinguished from E. crassipes by its rooted habit, and leaves that occur on the water’s surface.
1.2.3. Variations at the infraspecific level
No varieties or subspecies are currently recognised within the species.
2. Distribution and status
2.1. Native range
Eichhornia crassipes is indigenous to tropical South America, first described from Brazil in 1823 by C.F.P. Martius. Its centre of origin is Amazonia, Brazil, with anthropogenic spread to areas such as Argentina, Venezuela and central South America and the Caribbean islands (Barrett and Forno Citation1982; Edwards and Musil Citation1975; Penfound and Earle Citation1948).
South America: Argentina, Brazil, Chile, Columbia, Costa Rica, Ecuador, French Guiana, Guyana, Peru, Suriname, Venezuela.
2.2. Introduced range
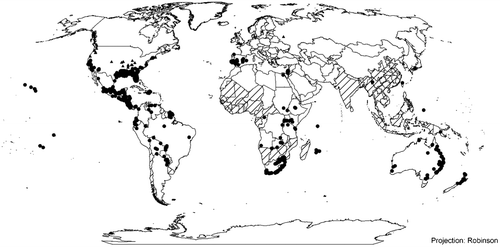
Eichhornia crassipes occurs on every continent except Antarctica, and in more than 50 countries, as the result of anthropogenic spread. Its distribution is largely restricted by cold winter temperatures to between 40°N and S, while it occurs abundantly in tropical freshwater bodies around the world (Figure ). Below is a list of invaded countries per global region:
Figure 3. Global distribution of Eichhornia crassipes, including established and casual populations. Where information has been provided by country, these administrative areas have been shaded. Where more precise distribution data is available this is indicated as dots, with established population indicated as circles, and ephemeral populations as triangles. Source: Kriticos and Brunel Citation2016 (with permission).
Asia: Bangladesh, Cambodia, China, Brunei Darussalam, India, Indonesia, Israel, Lebanon, Japan, Jordan, Laos, Malaysia, Maldives, Myanmar, Philippines, Singapore, South Korea, Sri Lanka, Syria, Taiwan, Thailand, Turkey, Vietnam.
North America: Mexico, U.S.A. Eichhornia crassipes has also been reported in ephemeral summer and autumn populations on the border of southern Canada (Ontario Province, tributaries to the southern side of Lake St. Clair and the Detroit River) (Adebayo et al. Citation2011). It is assumed that the finding of water hyacinth in these locations is due to repeated re-invasion from anthropogenic release by humans, rather than propagation by seed.
Central America: Guatemala, Honduras, Nicaragua, Panama.
Caribbean: Bahamas, Cuba, Dominican Republic, Haiti, Jamaica, Puerto Rico.
Oceania: American Samoa, Australia, Cook Islands, Fiji, French Polynesia, Guam, Marshall Islands, Federated States of Micronesia, Nauru, New Caledonia, New Zealand, Northern Mariana Islands, Palau, Papua New Guinea, Samoa, Solomon Islands, United States minor outlying islands, Vanuatu.
Africa: Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Congo, Côte d’Ivoire, Democratic Republic of Congo, Egypt, Equatorial Guinea, Ethiopia, Gabon, Ghana, Guinea, Guinea Bissau, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Niger, Nigeria, Reunion, Rwanda, Senegal, Sierra Leone, South Africa, Sudan, Swaziland, Tanzania, Togo, Uganda, Zambia and Zimbabwe.
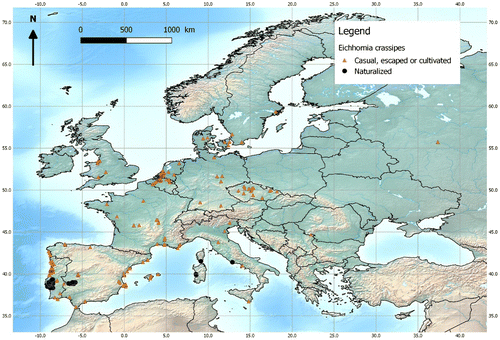
Europe: Established populations in Portugal, Spain, Italy and France (Q-Bank Invasive Plants Citation2017) (Figure ). In the Paúl do Boquilobo Biosphere Reserve in Central Portugal, it forms dense floating mats over extensive areas of wetlands and is considered the most obvious threat to the ecosystem. It is a permanent but controlled invasive aquatic weed in the irrigation canals, rice fields and riverine habitats of the Sado and Soraia River Basins, near Lisbon and the Atlantic Ocean, but is limited in the estuaries of these systems because it does not tolerate high salinity (Ruiz Téllez et al. Citation2008). The plant is also recorded as a casual invasive in Asturias, Huelva, Málaga, Cáceres, Taragona, Castellón, Alicante (Ruiz Téllez et al. Citation2008) and Valencia (Peña Bretón and de la Cruz Citation2014) in Spain. In 2005, it was reported to cover 75 km (approximately 200 ha) of the Guadiana River in the South Western Iberian Peninsula (Ruiz Téllez et al. Citation2008), which has since increased in extent to the Spain-Portugal border (Ruiz Téllez et al. Citation2016). There are also recent records of its invasion in Italy in Sardinia and Lazio (Brundu et al. Citation2012), while in other parts of the country (Campania, Tuscany, Sicily, Veneto), it is considered as a casual alien (Brundu et al. Citation2013) (Figure ). In France, the species is only naturalised in Corsica and has not spread (Tison and de Foucault Citation2014). In France mainland, the species has been increasingly recorded as escaped in the wild, in the west, the south-west, the Mediterranean region, and more rarely elsewhere (e.g. Georges and Pax Citation2002), but populations cannot tolerate continental winters (Fried Citation2017). In addition, it is recorded as a casual in several European countries with temperate climates, e.g. Belgium (Verloove Citation2006) Germany (Buttler, Thieme, and Mitarbeiter Citation2017), the Netherlands, the U.K. (Q-Bank Invasive Plants Citation2017) and the Czech Republic (Kaplan et al. Citation2016; Pyšek et al. Citation2012).It is also listed as present in Hungary and Romania (DAISIE Citation2008). The species was recorded in Moscow (Russia) but did not thrive. The species is also known to occur in thermally abnormal waters in Russia and Germany, e.g. the River Erft (Hussner and Lösch Citation2005), where it would normally be excluded due to cold winter temperatures.
Figure 4. Distribution map of Eichhornia crassipes in Europe. The map is given according to GBIF.org (Citation2017) with corrections based on our expertise on the species. Kaplan et al. (Citation2016) was used for distributon in Czech Republic; for France, we used the système d’information « Flore, Fonge, Végétation et Habitats » de la Fédération des Conservatoires botaniques nationaux (2016); Brundu et al. (Citation2012, Citation2013), Lastrucci and Foggi (Citation2006), Masin and Scortegagna (Citation2012) and Stinca, D’Auria, and Motti (Citation2012) were used for Italy; and Ruiz Téllez et al. (Citation2008, Citation2016) and Peña Bretón and de la Cruz (Citation2014) for Portugal and Spain. Black dots denote established populations, and orange triangles denote casual, escaped or cultivated populations. It is unlikely that all casual and cultivated records appear on this map, but, where present, they provide useful information showing where the species has escaped from cultivation or could potentially do so (propagule pressure). This information could be used along with the modelled potential distribution of Eichhornia crassipes under current climate conditions (Figure ). Map prepared by Guillaume Fried.
2.3. History of introduction and spread
The main mode of spread of water hyacinth is anthropogenic, via horticultural and aquarium trades due to the appeal of its beautiful flowers, and attractive smooth, green foliage and ease of cultivation. It continues to be introduced and spread through this pathway. The first authentic record of E. crassipes outside South America was from a trade fair in New Orleans in 1884 (Penfound and Earle Citation1948). Visitors to the cotton exposition were given plants as souvenirs by Japanese delegates, and many of these plants found their way into the waters of Louisiana, Texas, and Florida (Klorer Citation1909). A particularly troublesome invasion was on the St Johns River in Florida in 1895, when gale force winds blew the plant 160 km up and down the river, creating expansive floating mats up to 40 km long. Thereafter, E. crassipes plants spread around the U.S.A., remaining most problematic in the southern States as well as California. By the end of the nineteenth century, the plant was recorded in Egypt, India, Australia and Java (Gopal Citation1987). Its distribution is limited to latitudes of 40°N and S, with most invasions sites in the tropics, but it also occurs in warm temperate regions of the world (Gopal Citation1987). Eichhornia crassipes was first recorded in Australia in 1894, and by 1900, it was well established throughout Queensland and New South Wales (Wright and Purcell Citation1995). Introductions into China occurred during the early 1900s, and it is now widely distributed in sixteen provinces in southern China largely as a result of increased eutrophication of Chinese water bodies (Jianqing et al. Citation2001). Even though E. crassipes was first introduced to the African continent in Egypt between 1879 and 1892, and in South Africa in 1910 (Edwards and Musil Citation1975), many invasions in Africa were first noticed only in the 1980s, and it continues to invade many waterways of Africa, despite regional bans on its transport, and the implementation of numerous control efforts (Navarro and Phiri Citation2000).
Eichhornia crassipes was thought to have been introduced into Europe in the 1930s into Portugal, where it has since spread over the central-west of the country through irrigation canals. More recent investigations into the literature, however, have revealed records of E. crassipes introduction into the U.K. from Trinidad between 1823 and 1825, and it has been cited as cultivated at Kew Gardens in 1851 (Hooker 1851 in Brundu et al. Citation2013). It also appears that the plant was cultivated at various Botanical Gardens throughout Europe in the early 1800s, such as the Paris Botanic Garden from 1829 (Desfontaines 1829 in Brundu et al. Citation2013), and in the Vienna and Amsterdam Botanic Gardens (Endlicher 1842; Miquel and Groenewegen 1857, in Brundu et al. Citation2013), while instructions on how to cultivate it were published in Spain in 1859 (Colmeiro 1859 in Brundu et al. Citation2013), and in Italy in 1924 (Vagliasindi and Masera 1924 in Brundu et al. Citation2013). Brundu et al. (Citation2013) have subsequently deduced that E. crassipes was in fact quite a common garden plant in Europe in the early 1800s, calling into question Penfound and Earle’s (Citation1948) claim that the first authentic record in its adventive range was in 1884 in the U.S.A. However, establishment of permanent populations of the plant in Europe is more recent and has been limited to the warmer Mediterranean regions of the Iberian Peninsula, Italy and Corsica.
3. Ecology
3.1. Response to abiotic factors
3.1.1. Climate
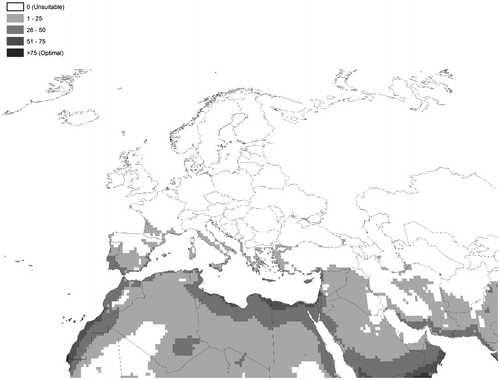
The distribution of E. crassipes is largely pantropical, with optimal growth occurring between 28°C and 30°C, while cold winter temperatures and frost events limit its spread (Owens and Madsen Citation1995). Eichhornia crassipes ceases to grow when water temperatures drop below 10°C (Gopal Citation1987), and during these times of stress, stored carbohydrates from the stem base are used as energy reserves (Owens and Madsen Citation1995). Eichhornia crassipes can withstand near-freezing temperatures for a limited period of time but exhibits a steady decline in regrowth potential under these conditions (Owens and Madsen Citation1995). However, during spring, regrowth of E. crassipes can occur from the crown of the plant, quickly resulting in new infestations under warmer climate conditions. Although prolonged cold weather may kill plants, the seeds remain viable (Ueki and Oki Citation1979) and allow regeneration when favourable conditions return. For these reasons, E. crassipes invasive range is restricted to the warmer Mediterranean regions in Europe, in Portugal, Spain, Italy and Corsica (France). However, according to future climate change projections (Figure ), the greatest potential for future range expansion lies in Europe. Countries at the greatest risk include Albania, Algeria, Bosnia and Herzegovina, Croatia, France (including Corsica), Greece, Israel, Italy (including Sardinia, Sicilia), Jordan, Montenegro, Portugal (including Azores and Madeira), Slovenia, Spain (including Baleares and Canary Islands), Turkey and Tunisia (Kriticos and Brunel Citation2016) (Figure ).
Figure 5. Modelled potential distribution of Eichhornia crassipes under current climate conditions in European and Mediterranean countries. It is assumed that Eichhornia crassipes will always be restricted to waterways within this climatically suitable envelope. Source: Kriticos and Brunel Citation2016 (with permission).
3.1.2. Waterbody types in native/invaded areas
Eichhornia crassipes invades still and slow-moving water bodies, resulting in thick extensive mats. It can tolerate pH levels between 4 and 10, but its proliferation prefers neutral pHs. These ranges do not limit its proliferation in most natural waters (Haller and Sutton Citation1973). It also thrives in fresh to brackish waters (up to 4 ppt) but is limited by very high salinity in coastal estuaries where concentrations between 6 and 8 ppt are lethal (de Casabianca and Laugier Citation1995; Muramoto, Aoyama, and Oki Citation1991), although there are reports that the plant may adapt its tolerance to higher salinities (de Casabianca and Laugier Citation1995).
By far the most significant factor affecting E. crassipes growth and proliferation is nutrient availability. Eichhornia crassipes growth is directly correlated with nutrient concentrations (Gopal Citation1987) – as nitrogen (N) and phosphorus (P) increase in concentration, so too does E. crassipes biomass accumulation (Gossett and Norris Citation1971; Reddy, Agami, and Tucker Citation1989, Citation1990). (See ‘4.2.1 Response to nutrient availability’ for more detail.)
3.2. Response to biotic factors
Eichhornia crassipes typically invades open waters, and because it is free-floating, it is not limited by depth of the water body. For this reason, it is able to outcompete littoral vegetation, forming dense uniform mats. Succession may follow by plants that use the floating mats of water hyacinth as a substrate. For example, in Lake Victoria, the succession of macrophytes increased dramatically after invasion by water hyacinth. After smothering other free-floating macrophytes like Pistia stratiotes L. (Araceae), native emergent macrophytes such as Ipomoea aquatica Forsk. and Enydra fluctuans Lour. invade the floating mat during early stages of succession. Thereafter, the rooted emergent hippo grass, Vossia cuspidate (Roxb.) Griff. (Poaceae), invades the floating mats at a later stage of succession, taking advantage of the anchorage and nutrients provided by the floating mats (Gichuki et al. Citation2012). This results in sediment accumulation, creating large floating sudds, at which point, E. crassipes biomass starts to decrease as it is shaded out. The floating islands of hippo grass subsequently decline, as the plants cannot extract nutrients from the water. Any surviving E. crassipes plants that survive beneath the mat may then float out into the open water, initiating new mats and starting the cycle all over again (Gichuki et al. Citation2012).
The ability of E. crassipes to multiply rapidly vegetatively confers significant competitive advantage over other aquatic macrophytes. It displays two phenologies – a short bulbous adventitious growth form that is prevalent at the leading edge of invasions, and a tall elongated growth form that dominates established mats. During invasion, as the density increases, the plants start vertical growth (elongation of petioles) together with an increase in leaf surface area (Center and Spencer Citation1981). The great morphological plasticity of the plant coupled with its wide ecological amplitude, which allows a high growth rate over a long period, also provide water hyacinth a competitive advantage over other free-floating macrophytes (Gopal and Goel Citation1993). For example, studies have demonstrated the superior competitive nature of E. crassipes when grown in culture with Pistia stratiotes L. (Agami and Reddy Citation1990; Center et al. Citation2005; Coetzee et al. Citation2005; Tag El Seed Citation1978). Eichhornia crassipes shades and stresses P. stratiotes plants through its high productivity and morphological plasticity (Agami and Reddy Citation1990).
3.3. Habitats and syntaxonomy
Eichhornia crassipes evolved in large, slow-flowing lowland rivers, such as the Amazon River, and the extensive marshes and lagoons of the Pantanal region in western Brazil, which were subject to regular hydrological cycles over long periods of time, where water levels fluctuate dramatically because of seasonal changes in rainfall. It has thus evolved to exploit such permanent and predictable water bodies. The Amazon River rises and falls about 10 m annually, as far as 2000 km upstream from its mouth. Under these conditions, rooted plants perish under submerged conditions, while free-floating species such as E. crassipes flourish.
In its native range, it usually occurs at relatively low densities, but becomes a problem where the hydrological regime of a water body has been altered by human activities, where the level of nutrients in the water has been increased, or where flushing of the plant and natural enemies occurs and the plant populations recovers faster than that of the natural enemies. Eventually the balance is restored as the populations of the natural enemies increase to reduce the plant populations (Julien, Griffiths, and Wright Citation1999), although there are some populations that require the use of other control methods under extreme human-mediated disturbance (Thomaz and Bini Citation1999).
In its invaded range, it invades still and slow-moving water bodies, forming thick impenetrable mats. It occurs in a wide range of aquatic habitats including estuarine habitats such as the Sacramento-San Joaquin River (Anderson Citation1990) and Mississippi Deltas (Penfound and Earle Citation1948) in the U.S.A., and the Nile Delta in Egypt (Navarro and Phiri Citation2000); lakes such as lakes Naivasha, Tanganyika, and Victoria in East Africa (Navarro and Phiri Citation2000); urban areas such as canals throughout Florida (Schmitz et al. Citation1991), water courses throughout the world, such as the Yangtze River in China (Gu Citation1991), the Hawkesbury River in Australia (Osmond and Petroeschevsky Citation2013), the Vaal River in South Africa (Cilliers Citation1991) and the Sepik River in Papua New Guinea (Orapa and Julien Citation2001); as well as numerous types of wetlands.
In Europe, water hyacinth has invaded a range of habitats, including the Pateira de Fermentelos, one of the largest natural freshwater lagoons in the Iberian Peninsula of Portugal (Laranjeira and Nadais Citation2008), the Guadiana and Júcar River Basins in Spain (Ruiz Téllez et al. Citation2008), and the river Mare’e Foghe, as well as the Pontine Marshes in Lazio, Italy (Brundu et al. Citation2012).
In all of these habitats, it can tolerate extremes of water-level fluctuation and seasonal variations in flow velocity, and extremes of nutrient availability, pH, temperature and heavy metals (Gopal Citation1987), but does not tolerate brackish and saline water (Muramoto, Aoyama, and Oki Citation1991) so is limited in estuarine habitats close to the ocean.
3.4. Ecological interactions
3.4.1. Herbivory
Due to the successful biological control programmes against E. crassipes in many parts of the world, herbivory in both the native and invaded ranges has been well researched (Cordo Citation1999; Perkins Citation1974). Initial surveys for phytophagous natural enemies were limited to the Amazon Basin, as this is considered the centre of origin of E. crassipes, with the highest diversity of co-evolved herbivores. Perkins (Citation1974) identified 43 herbivorous insects associated with water hyacinth in the Amazon, of which 19 were identified as inflicting sufficient damage, and potentially host-specific to be considered biological control agents. Perkins divided the type of damage into four categories: (1) defoliators and external leaf feeders, such as grasshoppers (e.g. Cornops spp.(Scuder 1875), (Orthoptera: Acrididae)), caterpillars (Lepidoptera), and the most well-known weevils, Neochetina eichhorniae and N. bruchi (Coleoptera: Curculionidae), which are the most widely used biological control agents throughout the tropics and subtropical areas of the world; (2) petiole borers, considered the most destructive herbivores as the result of subsequent waterlogging, such as the moth larvae (e.g. Niphograpta albiguttalis, and Xubida infusellus (both Lepidoptera: Crambidae), and Bellura densa (Walker 1865) (Lepidoptera: Noctuidae), as well as boring by the Neochetina spp.(Hustache 1926) larvae; (3) leaf tunnellers, with a single representative, the mite, Orthogalumna terebrantis (Sarcoptiformes: Galumnidae), specific to the Pontederiaceae; and (4) scavenger species, which enhance the attack by other species, but are not specific to water hyacinth. The most well-represented members are scarab beetles in the genera Dyscinetus (Harold 1869), Chalepides (Casey 1915) and Cyclocephala (Dejean 1821) (Coleoptera: Scarabaeidae), which feed inside the petioles or the crown, or at the base of the petioles, resulting in rotting of the plant material.
Because of the long history of exploration for natural enemies in South America, the discovery of additional species was thought unlikely; however, 30 years later, surveys conducted in 1999 and 2000 by the USDA (U.S.A. and Argentina), CABI (U.K.) and ARC-PPRI (South Africa) near Iquitos, Peru, at the confluence of the Marañon and Ucayali rivers (04°19ʹ29ʺS 73°18ʹ11ʺW), found a greater abundance and diversity of natural enemies on water hyacinth there than anywhere else on the continent, including most of the arthropods previously known to be associated with water hyacinth, and a range of new fungal isolates (Cordo Citation1999; Evans and Reeder Citation2001). These herbivores inflict a range of additional types of damage to E. crassipes, including flower feeding (e.g. Brachinus sp. (Weber 1801) (Coleoptera: Carabidae)), oviposition damage (e.g. Megamelus scutellaris (Hemiptera: Delphacidae)) and sap-sucking (e.g. Eccritotarsus catarinensis (Heteroptera: Miridae)).
Sufficient damage from herbivores can reduce biomass and vegetative reproduction, limiting population growth, under low-nutrient conditions and tropical to subtropical climates. However, results from biological control programmes around the world have shown that eutrophication of water bodies and cold winter temperatures limit the impact caused by herbivory (Akers, Bergmann, and Pitcairn Citation2017; Coetzee and Hill Citation2012; Hill and Olckers Citation2001).
In addition to arthropod herbivory, water fowl and livestock inflict sporadic damage to E. crassipes. Hippopotamus (Hippopotamus amphibius (Linnaeus 1758)) are also known to graze on the plant in infestations in Africa, and in 1910, a bill was almost passed in Louisiana, U.S.A., to introduce hippopotamus from Africa to eat E. crassipes and, at the same time, provide meat for the growing population (Miller Citation2013). Manatees (Trichechus manatus (Linnaeus 1758)) also include E. crassipes in their diet, where it is invasive in Florida, and E. crassipes has even been proposed as a diet supplement to increase this endangered species’ population numbers (https://www.conservationmagazine.org/2014/03/water-hyacinth-in-kings-bay/).
3.4.2. Plant pathogens
Various studies have been carried out around the world to isolate, identify and measure the pathogenicity of the fungi associated with E. crassipes in its native range, as well as in several infested areas of the world (Aneja, Srinvas, and Manpreet Citation1993; Bateman Citation2001; Charudattan Citation1996, Citation2001; Dagno Citation2006, Citation2011; El-Morsy Citation2004; Evans and Reeder Citation2001; Freeman, Charudattan, and Conway Citation1981; Kusewa Citation2002; Ray and Hill Citation2012; Ray, Sushilkumar, and Pandey Citation2008; Shabana, Charudattan, and Elwakil Citation1995a, Citation1995b; Tegene et al. Citation2012). Of the 60 fungi reported around the world, ten have been found to be highly virulent and known to generate diseases on E. crassipes. These fungi are: Acremonium zonatum (Sawada) W.Gams, Alternaria alternata (Fr.) Keissler, Alternaria eichhorniae Nag Raj & Ponnappa, Bipolaris spp., Cercospora piaropi Tharp. (=Cercospora rodmanii Conway), Fusarium chlamydosporum Wollenw & Reinking, Helminthosporium spp., Myrothecium roridum Tode ex Fr., Rhizoctonia solani Kühn and Uredo eichhorniae Gonz.-Frag. & Cif. (Charudattan Citation1996; Shabanna Citation2005). In Africa, priority was given to developing A. eichhorniae, A. zonatum, C. piaropi, R. solani, A. alternata and M. roridum as mycoherbicides for the control of water hyacinth (Bateman Citation2001).
3.4.3. Pollination
Although the major form of reproduction in E. crassipes is vegetative propagation, it still retains the potential for sexual reproduction in many regions of the world (Barrett Citation1980). The flowers of E. crassipes are tristylous, but unlike some other tristylous species, there is no incompatibility between the different forms (Barrett Citation1977). Flowers in both the native and introduced ranges are characterised by the predominance of a single style form, a condition atypical for heterostylous species. In most of the introduced range of E. crassipes, the midstyled form predominates, with long-styled forms occurring less frequently, while the short-styled form is restricted to the native range. The three floral forms of E. crassipes are highly self-compatible, and within style forms there is little difference in seed production between self and arthropod pollinations. In a study in the native range, the major insect visitor to flowers of E. crassipes was the bee, Ancyloscelis gigas (Friese 1904), while in the introduced range, the honeybee, Apis melifera (Linnaeus, 1758), is the dominant pollinator (Barrett Citation1980). An inflorescence with 20 flowers can produce over 3000 seeds, and up to four inflorescences can be produced by a single rosette during a 21-day period (Barrett Citation1980).
3.4.4. Dispersal
Water flow is the main mode of natural dispersal of E. crassipes within a waterbody. Daughter plants break off from the parent plant and are moved downstream, while seeds are considered hydrochorous, dispersed by rain wash, downstream flow and floods (Albano Pérez, Ruiz Téllez, and Sánchez Guzmán Citation2011). In addition, wind moves the plants around systems, too, creating large mats in the direction of the prevailing winds. Birds and mammals may disperse plants within and between water bodies; for example, hippopotamus are known to move plants large distances over land (Figure ). In addition, seeds may be transported over longer distances by birds and mammals when caught up in mud.
Figure 6. Hippopotamus appear out above an infestation of Eichhornia crassipes on the Mkhadzi River, in the Kruger National Park, South Africa. Hippopotamus are known to disperse plants within and between waterbodies. Photograph: J. Coetzee.

However, the main vector of dispersal is humans, through accidental or intentional spread. Recreational boaters and fishermen may inadvertently transport the plant in trailers or boat hulls between water bodies, while subsistence fishermen and recreational anglers are known to move plants around to increase habitat for fish fry (Hill Citation2003). However, it is the high ornamental value of the plant that has led, and still does lead, to its intentional introduction to water features around the world. Despite bans on its sale, it is still available directly from outlet stores or internet suppliers (Figure ) (Martin and Coetzee Citation2011; Padilla and Williams Citation2004).
4. Biology
4.1. Phenology
The growth form of E. crassipes plants is sympodial, and individual rosettes produce clones/ramets whose stolons decay or break once they have developed roots, separating them from the mother plant. These daughter plants form extensive floating mats supporting canopies that, in mature stands, extend a metre or more above the water surface (Center and Spencer Citation1981). These populations increase rapidly through the spread of these plants, being able to double their numbers under suitable conditions in one to two weeks (Edwards and Musil Citation1975), dependent on water nutrient content and temperature. In the Guadiana River in Spain, the doubling time varied between 10 and 60 days (Ruiz Téllez et al. Citation2008). Gopal (Citation1987) reviewed doubling times and showed them to vary from 5.9 to 28.1 days for weight, and from 3.7 to 57.8 days for numbers of plants as measured in the open (outside ponds) or in the field. At very high densities, self-thinning (density declines and biomass increases) regulates density (Center and Spencer Citation1981; Madsen Citation1993). Knowing the growth rate of plants in an area to be controlled and the condition that encourage growth is important for some control techniques (Julien Citation2008).
Eichhornia crassipes is also capable of sexual reproduction through the production of flowers and seeds. When flowering on a spike is complete, the flower stalk bends so that the inflorescence drops into the water, where the fruits split and seeds are carried away by flow, and eventually settle in the substrate. The seeds are capable of germinating immediately but may remain dormant for many years (Gopal Citation1987). Germination is encouraged by aerobic conditions and alternating temperatures; large populations of seedlings may become established on exposed mud at the edges of water bodies when water levels fall. Seedlings are rooted in mud initially, and after a short period of growth under water, they pop to the surface and float (Wright and Purcell Citation1995), becoming free-floating as a result of wave action or rising water levels. Seeds are the source of new infestations or re-invasions (Pieterse Citation1978), as are vegetative propagules. In the Iberian Peninsula, flowering occurs from June to October, and fruiting lasts until November (GIC Citation2006).
4.2. Physiology
4.2.1. Response to nutrient availability
Studies have shown that in nutrient-rich sites, water hyacinth biomass can increase eightfold compared with sites that are nutrient-poor (Reddy, Agami, and Tucker Citation1990), while increasing concentrations of nitrogen and phosphorus result in increases in ramet production, shoot/root ratio and plant height (Reddy, Agami, and Tucker Citation1989, Citation1990). Importantly, water phosphorus content significantly affects growth and nutrient storage by water hyacinth. All measures of water hyacinth growth increase with increasing phosphorus, but the rate of increase is not proportional (Reddy, Agami, and Tucker Citation1990). Although E. crassipes can grow in nutrient-poor (oligotrophic) waters, such as the Upper Shire River in Malawi, the plants do not grow well and are seldom problematic (Hill et al. Citation1999). On the other hand, eutrophication of Hartebeespoort Dam in South Africa resulted in a massive E. crassipes infestation during the 1970s and 1980s, which was subsequently reduced through chemical control alone (Ashton et al. Citation1979).
Eichhornia crassipes growth responds positively up to an N concentration of 5.5 mg/l and P concentration of 1.06 mg/l, but biomass accumulation does not significantly increase above these levels (Reddy, Agami, and Tucker Citation1989, Citation1990). However, N storage in the plant increases up to a maximum at 50.5 mg/l (Reddy, Agami, and Tucker Citation1989), while P storage in the plant increases indefinitely as the concentration of P in the water increases (Reddy, Agami, and Tucker Citation1990). Yet, net N storage within the plant is at a maximum when water P concentration reaches 2.56 mg/l (Reddy, Agami, and Tucker Citation1990), and net P storage is at a maximum when water N concentration is 5.5 mg/l (Reddy, Agami, and Tucker Citation1989). These N and P concentrations fit into the hypertrophic category of trophic terminology according to the Organization for Economic Co-operation and Development (Vollenweider and Kerekes Citation1982), reinforcing the fear that water bodies with excessive nutrient loading are at risk to E. crassipes invasion. In short, the availability of N in the culture medium affects the uptake of both N and P in the plant, as does the availability of P, suggesting that the N: P ratio in the water affects the N and P use efficiency by the plants. Haller and Sutton (Citation1973) found that if the phosphorus concentration falls below 0.1 mg/l, active growth of water hyacinth is halted, but concentrations above this allow for growth as well as the uptake of nutrients in excess of the plant’s requirements.
4.2.2. Response to flooding and drought
Eichhornia crassipes evolved traits to survive in its native habitat where desiccation and flooding are regular occurrences. It has tolerance to desiccation once water has receded, leaving the plants exposed on mud, and because it is free-floating and mobile, it is capable of surviving and flourishing on variable water levels. Germination occurs when substrates are exposed as water recedes and also as dry substrates are moistened when water levels rise. Seeds also survive in wet mud and are long-lived (Center and Spencer Citation1981), and flowers can be produced within 10–15 weeks after germination (Barrett Citation1980).
Therefore, in systems prone to flooding and drought, unscheduled, sporadic removals of E. crassipes result in resurgence from dormant seed banks and subsequent proliferation (Hill and Olckers Citation2001). The inflow of nutrient-rich water following flooding events is also key in stimulating E. crassipes production, as evidenced on the Paraná River floodplain in its native range (Neiff, Neiff, and Casco Citation2001). Recently, during times of drought in the Sacramento Delta, U.S.A., lower water flows and an influx of nutrients from agriculture and treated municipal sewage that was not flushed out of the system have resulted in the proliferation of E. crassipes (Anderson Citation2014; Khanna et al. Citation2012).
4.3. Reproductive biology
Eichhornia crassipes’ showy flowers are pale blue or violet, with a central yellow patch, and are borne on spikes. Flowers display the genetic polymorphism of tristyly in which all flowers of an individual plant possess one of three distinct corresponding style and stamen length phenotypes (Eckenwalder and Barrett Citation1986). Tristylous plants are usually self-incompatible, where very few seeds are produced as a result of self-pollination and pollination by plants of the same morph. However, E. crassipes defies this trend, because high levels of seed fertility are achieved in single morph colonies. The intermediate-style form of E. crassipes is prevalent in its introduced range, whereas the long-styled form occurs less frequently. The short-style forms predominate in restricted areas of its native range in South America but have not been recorded in its introduced range probably because of its relationship with a local pollinator, the long-tongued bee, Ancyloscelis gigas (Apidae) (Barrett Citation1977; Barrett and Forno Citation1982). Apis mellifera is documented as a pollinator in Argentina with potential economic importance to beekeepers, especially in coastal regions in the late summer and autumn when flowering terrestrial plants may be low in abundance (Fagúndez, Reinoso, and Aceñolaza Citation2016). In Europe, it is also pollinated by Apis mellifera (Ruiz Téllez et al. Citation2008).
When flowering on a spike is complete, the flower stalk produces narrow fruits, made up of three-celled capsules, 1–1.5 cm long. The number of mature fruits per flower spike is variable, and the number of seeds per capsule is also variable, 3–452. Seeds are oval and 1–1.5 mm long with longitudinal striations (Gopal Citation1987). A single E. crassipes inflorescence with 20 flowers can produce up to 3000 seeds, which are released in capsules that can accumulate in the floating mat or sink into the sediment below (Cronk and Fennessy Citation2001). Estimates of the number of seeds per square metre of vegetation range from 400 to 3400, depending on the sampling site and time of year (Cronk and Fennessy Citation2001; Pieterse and Murphy Citation1993). Seed banks are reportedly long-lived, remaining viable up to 20 years in sediments (Gopal Citation1987; Matthews Citation1967), and dormancy can be broken by wetting, drying and re-wetting (Baskin, Baskin, and Chester Citation2003). Seed bank persistence is therefore a significant factor influencing its eradication (Cacho et al. Citation2006) and long-term control. Despite these reproductive traits, which give E. crassipes an enormous potential for seed production, the majority of individuals in natural populations are probably produced by clonal growth (Barrett Citation1980). Sexual reproduction is most likely restricted in its introduced range by a scarcity of suitable pollinators, which limits fecundity, and unsuitable ecological conditions for seed germination and establishment (Barrett Citation1980).
Seed biology and germination conditions have been studied by Albano Pérez, Ruiz Téllez et al. (Citation2011) and it is influenced by the physicochemical composition of the water. This study investigated seed bank dynamics in South Africa, which has had a long history of invasion with those in Spain where the weed has been present for a far shorted period of time. Soil seed density varied between 0 and 2534 seeds/m2 but did not differ significantly between the type of waterbody (impoundment vs. river) or the history of control carried out at a site. Average germination was 54.17% and maximum germination around 3 days. The results from this study indicated that a combination of factors such as water fluctuation, eutrophication and seed decomposition might have had a great influence on dispersal and persistence of seeds.
5. Impacts
5.1. Uses and positive impacts
One hectare of E. crassipes may contain more than two million individual plants with a total wet weight of over 300 tonnes (Center and Spencer Citation1981), and it is this sheer biomass of plant material that has provoked research into its utilisation (Julien, Griffiths, and Wright Citation1999). Proposed uses for the weed include biogas production (Harley Citation1990), waste-water treatment, water-quality management, animal fodder, fertiliser, and the manufacture of paper and furniture (Julien, Griffiths, and Wright Citation1999). However, these industries all require investments and technological skills that would impose problems in developing countries where E. crassipes is often found, and there are risks of re-infestations from many of these uses (Albano Pérez et al. Citation2015). Nonetheless, there are numerous examples of utilisation in the developing countries of Africa and south east Asia, from cottage industries to internationally funded programmes aimed at minimising its impact on local communities. As an example, a German organisation promotes manufacturing furniture from E. crassipes in Thailand for sale in Germany (https://www.waterhyacinth.de/). Eichhornia crassipes is also widely available as an ornamental plant for garden ponds in Europe (PPP-Index Citation2017), for example, Germany (Hussner, Nehring, and Hilt Citation2014) and the U.K. (Figure ).
Eichhornia crassipes has been promoted as a relatively cheap and environmentally friendly tool for the decontamination of wastewater because of its rapid growth rate and high rate of heavy metal and nutrient absorption since the 1940s (Penfound and Earle Citation1948), and even today is used to treat contaminated water, particularly in Asian countries (Yan, Song, and Guo Citation2017). More recently, it has been used to prepare elastic cellulose-based aerogels with excellent oil sorption capacities, and reusability, which is very promising for cleaning oil spills (Yin et al. Citation2017). Sindhu et al. (Citation2017) discuss new value added products and fuels which can be produced from water hyacinth, while its use in the production of supercapacitors, and to improve the immune resistance of plants and animals is promising (Guna et al. Citation2017; Wu et al. Citation2016).
However, the biggest factor mitigating against its utilisation is its very high water content (on average 95%) (Harley Citation1990). To gain 1 tonne of dry material, 9 tonnes of fresh material has to be collected (Julien et al. Citation1996), making the cost of drying for the paper and furniture industries not commercially viable (Julien, Griffiths, and Wright Citation1999). In addition, E. crassipes as fodder for horses and cattle is inferior, again due to its high water content, and it is also unpalatable due to the high potash and chlorine content (Edwards and Musil Citation1975). Therefore, utilisation of E. crassipes is not feasible as a control method due to the low demand for E. crassipes products, the inaccessibility of most E. crassipes infestations and the high cost of processing the raw material (Julien et al. Citation1996) (see 6.2. below). For these reasons, E. crassipes utilisation will remain restricted to small-scale cottage industries, which are highly unlikely to provide a viable method for controlling or managing the weed. Consideration of possible utilisation of E. crassipes should therefore not prevent the execution of control programmes against it. In addition, a reliance on E. crassipes should not be created, because this would in turn create a conflict of interest and lead to an increase in the spread of the weed.
5.2. Negative impacts
5.2.1. On biodiversity and ecosystem functioning
Eichhornia crassipes is regarded as the world’s worst aquatic weed due to its documented socio-economic impacts, and its significant ecological impacts on aquatic ecosystem functioning. From an ecological perspective, drifting mats scour vegetation, destroying native plants and wildlife habitat. Eichhornia crassipes also competes with other plants, often displacing wildlife forage and habitat (Center et al. Citation2002). Increased detrital production and siltation occur under E. crassipes mats due to high sediment loading. Dense mats reduce light to submerged plants, thus depleting oxygen in aquatic communities (Mitchell Citation1985; Rommens et al. Citation2003; Ultsch and Anthony Citation1973). The resultant lack of phytoplankton (McVea and Boyd Citation1975) alters the composition of invertebrate communities (Brendonck et al. Citation2003; Hansen, Ruby, and Thompson Citation1971; O’Hara Citation1967). For example, Masifwa, Twongo, and Denny (Citation2001) showed an increase in macroinvertebrate abundance at the edges of E. crassipes mats on Lake Victoria, Uganda, while Midgley, Hill, and Villet (Citation2006) and Coetzee, Jones, and Hill (Citation2014) showed that E. crassipes mats significantly reduced the diversity and abundance of benthic invertebrates in impoundments in South Africa. In Spain, GIC (Citation2006) reported significant reductions in the total density of phytoplankton, and zooplankton after invasion by E. crassipes. Changes in zooplankton communities were evidenced by the substitution of crustacean species for others (e.g. Tropodiaptomus processifer (Kiefer 1926), T. kaepelini (Poppe and Mrázek 1895) and T. orientalis (Brady 1886) by Ceriodaphnia dubia (Richard 1894), and Daphnia dubia (Herrick 1883)). In southern Europe, E. crassipes outcompetes a number of aquatic plant species, including species in the Potamogeton L., Ranunculus L., Myriophyllum L., Nuphar Sm., Nymphea L. and Zanichellia L. genera (GIC Citation2006). Elimination of these species alters the habitat of aquatic communities, effecting changes in aquatic biodiversity.
Effects of E. crassipes infestations on fish abundance and diversity depend on its impacts on invertebrate and plankton abundance and diversity as these are crucial links in the trophic ecology of aquatic ecosystems. Fish abundance and diversity will respond positively to an increase in epiphytic invertebrate community increases, for example, in Lake Chivero in Zimbabwe, E. crassipes mats generally have a positive effect on taxon diversity of fishes by providing shelter and feeding grounds for small fishes (Brendonck et al. Citation2003). Similarly, the increase in macroinvertebrate abundance at the edges of mats in Lake Victoria resulted in Nile tilapia shifting their diets to include a larger component of these species (Njiru et al. Citation2004). Conversely, a decrease in phytoplankton as a result of shading and increased turbidity may decrease dissolved oxygen concentrations and planktivorous fish abundance, subsequently affecting higher trophic levels.
Water bird communities also change in the presence of E. crassipes infestations. At moderate densities, bird communities may benefit if macroinvertebrate and fish abundances increase. In Florida, birds stalking in E. crassipes mats most often obtained prey located near the perimeter of mats, and rarely hunted for food in the interior of mats (Bartodziej and Weymouth Citation1995). However, when water hyacinth becomes dominant, outcompeting other vegetation types and preventing access to water, water bird diversity may be negatively affected when dense mats physically prevent water bird access to prey, or if dissolved oxygen reductions cause negative effects on prey populations (Villamagna and Murphy Citation2010).
5.2.2. Other impacts
In addition to the biodiversity impacts, the socio-economic impacts of E. crassipes have also been well documented. Dense impenetrable mats restrict access to water, negatively impacting fisheries and related commercial activities, the effectiveness of irrigation canals, navigation and transport, hydroelectric programmes, and tourism (Navarro and Phiri Citation2000). For example, the hydropower station at the Kafue Gorge Dam in Zambia is responsible for supplying 900 MW of power to the country. At the height of the E. crassipes problem on the dam, at least one of the five turbines was forced to be shut down for a day per week. This was due to the increased concentration of nitrous oxides in the water that caused a certain amount of corrosion on the turbines. The hydropower dams on the Shire River in Malawi and the Owen Falls Dam at Jinja in Uganda on the Nile River are also frequently forced to stop production due to E. crassipes clogging the intakes for the water cooling system. No estimates of costs of this are available, but it must amount to several million USD per year (Wise et al. Citation2007). The impact of the plant in 2007/2008 on the Victoria Falls Power Station production of electricity amounted to US$946,822 (Nang’alelwa pers. comm. 2008). Other problems include property damage during floods as a result of E. crassipes building up against bridges, fences, walls, obstructing water flow and increasing flood levels. Arguably, the most affected are poverty-stricken communities in rural Africa, where the extent of these effects are yet to be fully measured. Eichhornia crassipes alters the livelihoods of any community with high dependence on freshwater waterways for food (subsistence or commercial), transport and clean water.
In many rice growing areas of the world, E. crassipes has hindered production through competition for nutrients, allelopathy and restricting accessibility, particularly in deepwater rice culture (Smith Citation1983). These problems have been reported from Bangladesh (Whitton et al. Citation1988), Sri Lanka (Room and Fernando Citation1992), and West Bengal in India (Datta and Banerjee Citation1978), as well as anecdotal evidence from Africa and South East Asian countries. In Europe, E. crassipes has invaded rice fields in Portugal (Figueiredo et al. Citation1984; Guerreiro Citation1976).
Eichhornia crassipes infestations intensify mosquito problems by hindering insecticide application, interfering with predators such as fish, increasing habitat for species that attach to plants, and impeding runoff and water circulation (Seabrook Citation1962). Despite there being numerous references attributing an increase in malaria to E. crassipes infestations, in one of the quantified surveys, Mailu (Citation2001) was unable to show a correlation between the explosion of E. crassipes on Lake Victoria and an increase in the disease. Eichhornia crassipes provides the ideal habitat for the snail vectors (Biomphalaria spp. (Preston 1910) and Bulinus spp. (O.F. Müller, 1801)) of the bilharzia schistosome, and there is some evidence from Ghana that increased infestations of E. crassipes are linked to an increase in the prevalence of this disease (Navarro and Phiri Citation2000). It also blocks access to water points and, as such, has been linked to an increase in cholera and typhoid (Navarro and Phiri Citation2000). Furthermore, E. crassipes harbours venomous snakes, crocodiles and hippos, making the collection of water dangerous, sometimes fatal (Gopal Citation1987; Navarro and Phiri Citation2000).
In Europe, E. crassipes has invaded irrigation canals fed by the Guadiana River reducing water supply to important fruit growing areas. Further, the weed in this river system has had an impact on cross-border tourism from Portugal as the river and the Spanish–Portuguese Alqueva Reservoir attracts many tourists annually, who do not wish to see the waterbodies covered by the weed (Ruiz Téllez et al. Citation2016).
6. Legislation and management
6.1. Legislation
Eichhornia crassipes is on the 100 of the World’s Worst Invasive Alien Species List. These 100 species are a selection from the Global Invasive Species Database, published by the Invasive Species Specialist Group (ISSG), a specialist group of the Species Survival Commission of the International Union for Conservation of Nature, as a contribution to the Global Invasive Species Programme. Many countries have legislation regulating E. crassipes: it is on the USDA/APHIS noxious weeds list, and it is a class 1 noxious weed in Australia, a prohibited weed in New Zealand, a declared invader in Botswana and a Category 1b weed in South Africa. All of these regulations prohibit the importation and sale of the weed and require land users and water authorities to control infestations in an appropriate manner. On the Asian continent, there are several pieces of legislation preventing the possession and trade of E. crassipes, but most of these date back to the early twentieth century, e.g. the Water Hyacinth Act of 1917, Burma (Gutter Citation2001), 1908 Act in China, Agricultural Pests and Diseases Act of 1919 in Madras, the Water Hyacinth Act of 1926 in Assam, and the Water Hyacinth Act of 1936 in Bengal (Iqbal Citation2010). Although no recent legislation is in place, this plant is still considered a threat, and governments consider it an unwanted non-native species.
After completion and approval of the EPPO PRA on E. crassipes, in 2008, the species was recommended for regulation within European and Mediterranean countries. Because of its negative impacts, the species is subject to various legal restraints. In addition to general rules concerning the use of alien plants like the prohibition to cause them to grow in the wild that is present in the nature conservation acts of several European countries, some legal texts mention the species explicitly: the recent EU Regulation No. 1143/2014 ‘on the prevention and management of the introduction and spread of invasive alien species’ (EU Citation2014) contains a catalogue of restrictions for the use of alien species. According to this regulation “invasive alien species of Union concern” may not be brought into the territory of the Union, kept, bred, transported to, from or within the Union, placed on the market, etc. Invasive species are assigned to this list on the basis of detailed risk assessments. The first list of 37 animal and plant species was published by the European Commission in July, 2016. It contains E. crassipes, which was listed based on the EPPO PRA (EPPO Citation2008). Commercial owners may continue selling listed species for one year after coming into force.
In Portugal and Spain, legal restrictions to the use of E. crassipes were in place before the EU Regulation based on national and/or communal laws, e.g. the Portuguese Decreto – Lei no. 565/99 of December, 1999; or the Spanish Real Decreto 630/2013 of August, 2013 (Ministerio de Agricultura, Alimentación y Medio Ambiente Citation2013).
6.2. Management
Several control methods have been implemented against E. crassipes, utilisation, manual removal and mechanical control, hydrological manipulation, the application of herbicides and biological control, and more recently attempts have been made to integrate these control methods (Hill and Coetzee Citation2008). The EPPO standard describes the procedures for control of E. crassipes in the EU (EPPO Citation2009). Unfortunately, this plant is well adapted to surviving the many procedures that have been used for aquatic weed management such as the removal or killing of plants, draw down or flushing downstream.
6.2.1. Utilisation
Eichhornia crassipes utilisation is not commercially viable (see above), and its utilisation should not be considered as a control option, nor should it prevent the implementation of other control programmes. In addition, a reliance on E. crassipes utilisation would create a conflict of interest and lead to further spread of the weed, as has already been observed in some areas in Africa (see Section 5.1 above for details).
6.2.2. Manual removal
Manual removal through hand pulling or using pitch forks is used in a number of regions of the world, most notably, southern Africa, Europe and China. This method is very labour intensive, only effective for small infestations and essentially used as an employment creation exercise where labour is relatively inexpensive (Fig. 9). Zimbabwe initiated a manual removal programme on Lake Chivero, in the early 1980s (Chikwenhere and Phiri Citation1999). The manual removal team consisted of 500 workers, working an 8-hour day. Although almost 500 tonnes of water hyacinth was removed, the rapid regeneration of the weed made the effort slow and expensive, with no obvious impact 6 months later. It was then decided to implement mechanical control using a bulldozer, a boat, a conveyor and dump trucks. Even though almost 2 ha of plants was cleared daily, neither manual removal nor mechanical harvesting effectively reduced the amount of water hyacinth on the lake (Chikwenhere and Phiri Citation1999).
6.2.3. Mechanical control
Mechanical control, through the use of harvesters, has been used in many parts of the world to control E. crassipes. Again, the amount of biomass that needs to be removed, coupled with the growth rate of the plant, and the remoteness of some of the infested areas means that this option has limited applicability. For example, harvesters have been used at Port Bell and Owen Falls Dam on Lake Victoria, Uganda with limited success (Mailu Citation2001) and on the Liwonde Barrage in Malawi. Despite these problems, reasonably successful results were obtained in Mexico, where a combined chemical-mechanical programme, using the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) and a mechanical harvester, was implemented to control water hyacinth on the Trigomil Dam (Gutiérrez et al. Citation1996). Furthermore, mechanical harvesting was successful on a series of small lakes in the Gauteng Province of South Africa, mainly because it was implemented in winter when the plant was not actively growing.
Despite the expense and limited applicability of mechanical harvesting, it is often the only solution available for the control of E. crassipes in rivers, impoundments and lakes in Europe. For example, some 8 km (560,000 m2) of the River Mare ‘e Foghe (central-eastern Sardinia) was covered by a dense mat of E. crassipes (mixed with Hydrocotyle ranunculoides L.f. (pennywort)) in 2010 (Brundu et al. Citation2012). Mechanical control was implemented using crane trucks with grapples and pushing boats. Sites that were difficult to access were cleaned using motor boats and manual extraction means, or boats equipped with cutting devices. By December 2010, some 6700 tonnes of plant biomass had been removed at a cost of €175,000. A 400–500 m (25,000 m2) stretch of river invaded by the mat of E. crassipes remained, and an additional €400,000–500,000 was set aside to continue operations up to 2013.
E. crassipes was first recorded in the Lazio region, in the Pontine plains, in 1983 (Anzalone Citation1983). In the early colonisation phases, the species did not outcompete other species (Scoppola, Iberite, and Palazzi Citation1986); and until 1995, its populations were still small, limited to a few sites on the shores of the Rio Martino river near the Fogliano lake, sometimes even in brackish waters. In the following years, the populations of E. crassipes spread to other sites within the Pontine plains, covering a total surface of 5,000 m2 in 2004 and 2005 (Iberite and Pelliccioni Citation2009). Every autumn, the weed is mechanically removed by the local authority responsible for water management (i.e. the Consorzio di Bonifica di Latina).
In 2006, mechanical harvesters were used to control E. crassipes in the lagoon system of Ria de Aveiro, Portugal that was approximately 50% covered by the weed. Since the operation began, the aquatic-harvester has removed more than 15,500 m3 of mats from the lagoon, which, in accordance with the current legislation, has been transported to an old inactive quarry site. At present the lagoon remains free of E. crassipes, allowing navigation and the maintenance of traditional activities such as fishing and boating (Laranjeira and Nadais Citation2008).
One of the most prominent cases of E. crassipes invasion and control in Europe comes from the Guadiana River in Spain (Figure ). The weed was first recorded on the river in 2004 (Ruiz Téllez et al. Citation2008). Measures carried out by Spain’s Ministry of the Environment managed to retain the infestation to a 75 km section of the river. Control of the weed relied on physical methods with manual and mechanised extraction and the installation of floating booms to prevent the spread of the infestation downstream. By 2008, €8 million had been spent and 2,000 tonnes of biomass had been extracted. However, an EPPO International Workshop on the topic highlighted the river as a high re-infestation risk area (Martín de Rodrigo et al. Citation2008). Eichhornia crassipes did reinfest the river, most likely from seed, or scattered plants that the mechanical harvesting had missed, and in 2010, an additional 5 tonnes of the weed was removed, more than 51,000 tonnes was removed in 2012, and 170,000 tonnes was removed in 2016. Thus, in ten years of control (2005–2015), up to €26,000,000 had been spent (Duarte Citation2017). Despite this effort, scattered populations of E. crassipes had spread along 150 km of the river, just about reaching Portugal and Alqueva, the largest Reservoir in Europe. Management had thus failed, so a group from diverse social sectors travelled to the European Parliament in 2015 to raise the alarm about the situation. Community Union later included E. crassipes in the list of Invasive Alien Species of Union concern that required the relevant authorities to reinforce control strategies.
Figure 8. Eichhornia crassipes and Nymphaea mexicana Zucc.on the Guadiana River near the border of Portugal, in Badajoz, Spain. Photograph: Asociación Ciudadana Salvemos el Guadiana.

Figure 9. Manual removal teams attempting to remove Eichhornia crassipes on the Vaal River, South Africa.

The above examples show that while mechanical control can be a useful tool in the control of E. crassipes, it is expensive and will not lead to the eradication of the weed, as it will recruit from a long-lived seed bank and thus requires a long-term commitment to this method (Ruiz Téllez et al. Citation2016). Furthermore, the remoteness of many infestations, and the shallowness of many of the invaded systems makes mechanical control unfeasible.
6.2.4. Hydrological manipulation
Water-level manipulation has been suggested for the control of E. crassipes. This method is appropriate for infestations on man-made impoundments where a drawdown of water is possible, stranding plans on the banks and allowing them to desiccate. Exposure of the sediments to sun, also allows for the top layer of soil containing seeds to be removed. Floods are very effective at controlling water hyacinth, especially in systems close to the ocean where salt water will kill the plants. Floods can be manipulated in river systems and canals downstream of impoundments, but only in areas where water scarcity is not an issue.
6.2.5. Herbicide application
The application of herbicides has been widely used in the control of water hyacinth throughout the world since the 1960s. Although herbicide application has the advantage of being fast acting, effective control depends on a long-term commitment to follow-up applications for possibly 20 years or more. Water hyacinth is very susceptible to herbicides such as 2,4-D, diquat, paraquat and glyphosate (Gopal Citation1987), which have resulted in successful control in small, single-purpose water systems such as irrigation canals and dams of around 1 hectare in size (Wright and Purcell Citation1995). In developing countries, many water hyacinth-infested sites are used for drinking water, for washing and for fishing, and so the use of chemical sprays contaminates these sites, and can threaten human health (Julien, Griffiths, and Wright Citation1999). The herbicide control of water hyacinth is often not appropriate in developing countries, as it is expensive and requires highly skilled personnel, and often herbicides are perceived as poisons. Furthermore, herbicides are not permitted for application onto waterways in Europe, so this control method is not an option against E. crassipes in the EU.
6.2.6. Biological control
It has often been suggested that biological control, or the release of host-specific natural enemies, is the only option that offers economical and sustainable control of the weed (Harley Citation1990). Research into the biological control of water hyacinth was initiated by the United States Department of Agriculture in 1961, and to date eight arthropod species including two weevils (Neochetina eichhorniae and Neochetina bruchi), two moths (Niphograpta albiguttalis and Xubida infusellus), three sap-sucking bugs (Eccritotarsus catarinensis, Eccritotarsus eichhorniae and Megamelus scutellaris), one grasshopper (Cornops aquaticum) and one mite species (Orthogalumna terebrantis) have been released to control water hyacinth in its adventive range (Coetzee et al. Citation2011; Paterson et al. Citation2016; Winston et al. Citation2014) (Table ). In addition, the fungal pathogen Cercospora piaropi (Tharp 1917) (Capnodiales) (Mycosphaerellales: Mycosphaerellaceae) has been released as a classical biological control agent for the weed in South Africa (Morris, Wood, and Den Breeÿen Citation1999). A programme (the International Mycoherbicide Programme for water hyacinth control in Africa (IMPECCA)), which was established to provide support for the discovery of a promising, acceptable and relevant fungi as mycoherbicides, ceased before any useful findings could be obtained (Ray and Hill Citation2012).
Table 1. Characterisation of major arthropods associated with Eichhornia crassipes (modified and updated from Center et al. (Citation2002)).
The arthropod agents have all been extensively studied and have been shown to be host-specific to the weed (Center et al. Citation2002). This control method has been widely accepted, and the two weevil species are the most commonly used with N. eichhorniae released in 39 countries, and N. bruchi in 36 counties around the world (Winston et al. Citation2014). The biological control of water hyacinth has been highly successful in a number of large tropical water bodies, mainly in Africa (Coetzee et al. Citation2011; Hill and Julien Citation2004), but also in Australia (Julien Citation2001) and Papua New Guinea (Orapa and Julien Citation2001).
Defining “control” and the length of time taken to achieve it is a fundamental issue in quantifying the benefits of control. Complete control of E. crassipes is considered to be reached when E. crassipes populations are reduced below an ecologically or economically viable threshold and are maintained at that threshold with no requirement of an additional intervention. Biological control is considered the most cost-effective method, but it takes a long time (3–5 years under ideal conditions), compared with manual control, which achieves instant success in a short period of time, but requires considerable human input to do so, and the results are not sustainable. Herbicidal control also achieves success in a short time period but is expensive, has negative environmental side effects and requires considerable follow-up.
Benefits relating to biological control of aquatic weeds have been assessed for Australia (Page and Lacey Citation2006). The costs of the biological control projects was approx. A$5 million in 1974–1993. The E. crassipes project cost A$636,000 in the period 1974–1991, and the combined cost–benefit ratio was 27.5:1. A much higher cost–benefit ratio was achieved for the biological control programme in southern Benin, due to the direct economic effects on the local people. At its peak of infestation, E. crassipes reduced the annual income of approximately 200,000 people by about US$85 million, compared with the total cost of the control programme of about US$2 million (in 1999 US$ accrued at 6% p.a., for a total duration of 20 years), yielding a cost–benefit ratio of 124:1 (De Groote et al. Citation2003).
The lack of clear government policies that permit the use of biological control agents of weeds is a major constraint for the management of E. crassipes in Europe. Nearly 40 countries around the world practice biological control of E. crassipes, often with great success. It is encouraging that interest and impetus to use biological control for weed management in Europe are increasing (Djeddour et al. Citation2008; Shaw Citation2008) with intentional releases in three countries over the past five years (Shaw, Schaffner, and Marchante Citation2016).
6.2.7. Integrated control
Despite biological control having been highly successful in some regions of the world, in others acceptable levels of control have not been achieved through this method, or biological control is perceived to be too slow acting. Thus an integrated management approach has been suggested for E. crassipes. An integrated management approach includes aspects of biological control, herbicide applications, manual removal and, possibly most importantly, the management of nutrients entering the aquatic ecosystem that drives E. crassipes growth (Hill and Olckers Citation2001). Jones and Cilliers (Citation1999) and Jones (Citation2001) developed an integrated management programme for the Nseleni River system, a subtropical region of South Africa, and this approach was further developed by Hill and Coetzee (Citation2008). The key elements of this approach, which is applicable worldwide but pertinent to the European scenario, were to appoint one individual or organisation to drive the control programme; involve all interested and affected parties on the water body, including citizen science and volunteer groups; map the extent of the infestation to understand how big the infestation is; identify the cause of the infestation to determine where the infestation is coming from and what is driving its invasion; set the acceptable level of control; use appropriate control methods where applicable; evaluate the level of success of the control options; and adjust the management plan over time, depending on the outcomes of the evaluation.
7. Conclusion
In 2008, Julien outlined the challenges for the control of E. crassipes in Europe, and the outlook has not changed considerably, despite recent EU regulation of the species. The plant is already widely established in southern Europe, southwest of the Iberian Peninsula, and given the reality of global climate change, more areas will become suitable for establishment. Attempts to eradicate it have largely failed, while control options are limited. Herbicides are not permitted on most waterways, biological control has not been legislated to date, manual and mechanical control has proven to be expensive and generally ineffective, and the plant is still traded within the borders of the EU. Even though the pathways for importation and dispersal within the EU have recently been regulated, and control options remain limited, E. crassipes is likely to become more problematic under current climatic conditions, and will no doubt increase its range under future climate change scenarios. Additionally, the plant thrives in eutrophic waters, which are typical of many European water bodies. Under the conditions outlined above, E. crassipes will become increasingly invasive and damaging in Europe, and until there is a change in legislation allowing for derogations for temporary herbicide use in waterways, and a more generalised use of classical biological control, prospects for the effective management of this weed remain poor. It is therefore crucial to address water quality and new introductions in the absence of effective control.
Notes on contributors
Julie Coetzee is Associate Professor of Botany at Rhodes University, with a research focus on aquatic weed invasions and their control. Contribution: drafted the whole review.
Martin Hill is Professor of Entomology at Rhodes University, and his research focuses on the integrated management of invasive species. Contribution: co-contributed to the drafting of the review, in particular legislation and management.
Trinidad Ruiz Téllez is Profesora Titular de Botánica at the Universidad de Extremadura. Contribution: provided additions and comments with regard to distribution, biology, phenology, impact and management of E. crassipes in Europe.
Uwe Starfinger is an invasive species ecologist with a background in PRA development, based at the Julius Kuehn Institute, Federal Research Centre for Cultivated Plants. Contribution: provided additions and comments with regard to Eichhornia crassipes in Europe.
Sarah Brunel is an agronomist with an interest in plant health; she currently works as Capacity Development Officer for the International Plant Protection Convention. Contribution: coordinated the EPPO PRA on Eichhornia crassipes while previously working for the European and Mediterranean Plant Protection Organization (EPPO), and provided comments on this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
We thank Guillaume Fried for his useful comments on the MS, as well as for developing the map of Eichhornia crassipes in Europe. We also thank the two anonymous reviewers for constructive suggestions on a previous version of the MS.
References
- Adebayo, A., E. Briski, O. Kalaci, M. Hernandez, S. Ghabooli, B. Beric, F. Chan, et al. 2011. “Water Hyacinth (Eichhornia crassipes) and Water Lettuce (Pistia stratiotes) in the Great Lakes: Playing with Fire?” Aquatic Invasions 6: 91–96.10.3391/ai
- Agami, M., and K. R. Reddy. 1990. “Competition for Space between Eichhornia crassipes (Mart.) Solms and Pistia stratiotes L. cultured in Nutrient-Enriched Water.” Aquatic Botany 38: 195–208.10.1016/0304-3770(90)90005-6
- Akers, R. P., R. W. Bergmann, and M. J. Pitcairn. 2017. “Biological Control of Water Hyacinth in California’s Sacramento-San Joaquin River Delta: Observations on Establishment and Spread.” Biocontrol Science and Technology 27: 755–768.10.1080/09583157.2017.1342220
- Albano Pérez, E., J. A. Coetzee, T. Ruiz Téllez, and M. P. Hill. 2011. “A First Report of Water hyacinth (Eichhornia crassipes) Soil Seed Banks in South Africa.” South African Journal of Botany 77: 795–800.10.1016/j.sajb.2011.03.009
- Albano Pérez, E., T. Ruiz Téllez, and J. M. Sánchez Guzmán. 2011. “Influence of Physico-Chemical Parameters of the Aquatic Medium on Germination of Eichhornia crassipes Seeds.” Plant Biology 13: 643–648.10.1111/plb.2011.13.issue-4
- Albano Pérez, E., T. Ruiz Téllez, S. Ramos Maqueda, P. J. Casero Linares, F. M. Vázquez Pardo, P. L. Rodríguez Medina, J. Labrador Moreno, F. López Gallego, J. González Cortés, and J. M. Sánchez Guzmán. 2015. “Seed Germination and Risks of Using the Invasive Plant Eichhornia crassipes (Mart.) Solms-Laub. (Water hyacinth) for Composting, Ovine Feeding and Biogas Production.” Acta Botanica Gallica 162: 203–214. doi:10.1080/12538078.2015.1056227.
- Anderson, L. W. J. 1990. “Aquatic Weed Problems and Management In Western United States and Canada.” In Aquatic Weeds: The Ecology and Management of Nuisance Aquatic Vegetation, edited by A. H. Pieterse and K. J. Murphy, 371–391. Oxford: Oxford University Press.
- Anderson, T. 2014. Water hyacinth Thrives in Drought Stricken Delta. https://baynature.org/article/water-hyacinth-thrives-drought-stricken-delta/
- Aneja, K. R., B. Srinvas, and K. Manpreet. 1993. “Evaluation of Fusarium chlamydosporium as a Biocontrol Agent of Water hyacinth (Eichhornia crassipes)(Mart.) Solms.” In Integrated Weed Management of Sustainable Agriculture: Proceeding of Third Indian Society of Weed Science International Symposium, edited by Indian Society of Weed Science, 145-149. Misar: Indian Society of Weed Science.
- Anzalone, B. 1983. “Notes on the Roman Flora: on several novel species found in Lazio.” Informatore Botanico Italiano 15: 13–17.
- Ashton, P. J., W. E. Scott, D. J. Steyn, and R. J. Wells. 1979. “Chemical Control Programme against the Water hyacinth Eichhornia crassipes (Mart.) Solms on Hartbeespoort Dam: Historical and Practical Aspects.” South African Journal of Science 75: 303–306.
- Barrett, S. C. H. 1977. “Tristyly in Eichhornia crassipes (Mart.) Solms (Water hyacinth).” Biotropica 9: 230–238.10.2307/2388140
- Barrett, S. C. H. 1980. “Sexual Reproduction in Eichhornia crassipes (water hyacinth). II. Seed Production in Natural Populations.” Journal of Applied Ecology 17: 113–124.10.2307/2402967
- Barrett, S. C. H., and I. W. Forno. 1982. “Style Morph Distribution in New World Populations of Eichhornia crassipes (Mart.) Solms-Laubach (Water hyacinth).” Aquatic Botany 13: 299–306.10.1016/0304-3770(82)90065-1
- Bartodziej, W., and G. Weymouth. 1995. “Waterbird Abundance and Activity on Water Hyacinth and Egeria in the St. Marks River, Florida.” Journal of Aquatic Plant Management 33: 19–22.
- Baskin, C. C., J. M. Baskin, and E. W. Chester. 2003. “Ecological Aspects of Seed Dormancy-Break and Germination in Heteranthera limosa (Pontederiaceae), a Summer Annual Weed of Rice Fields.” Weed Research 43: 103–107.10.1046/j.1365-3180.2003.00321.x
- Bateman, R. 2001. “IMPECCA: An International, Collaborative Program to Investigate the Development of a mycoherbicide for use Against Water hyacinth in Africa.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 57-61. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Brendonck, L., J. Maes, W. Rommens, N. Dekeza, T. Nhiwatiwa, M. Barson, V. Callebaut, et al. 2003. “The Impact of Water hyacinth (Eichhornia crassipes) in a Eutrophic Subtropical Impoundment (Lake Chivero, Zimbabwe). II. Species Diversity.” Archiv für Hydrobiologie 158: 389–405.10.1127/0003-9136/2003/0158-0389
- Brundu, G., A. Stinca, L. Angius, G. Bonanomi, L. Celesti-Grapow, G. D’Auria, R. Griffo, A. Migliozzi, R. Motti, and P. Spigno. 2012. “Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms.: Emerging Invasive Alien Hydrophytes in Campania and Sardinia (Italy).” EPPO Bulletin 42: 568–579.10.1111/epp.2012.42.issue-3
- Brundu, G., M. M. Azzella, C. Blasi, I. Camarda, M. Iberite, and L. Celesti-Grapow. 2013. “The Silent Invasion of Eichhornia crassipes (Mart.) Solms. in Italy.” Plant Biosystems 147 (4): 1120–1127.10.1080/11263504.2013.861536
- Buttler, K. P., M. Thieme, and Mitarbeiter. 2017. Florenliste von Deutschland [Flora List of Deutschland]. Accessed August 31, 2017. https://www.kp-buttler.de/florenliste/.
- Cacho, J. O., D. Spring, P. Pheloung, and S. Hester. 2006. “Evaluating the Feasibility of Eradicating an Invasion.” Biological Invasions 8 (4): 903–917.10.1007/s10530-005-4733-9
- de Casabianca, M.-L., and T. Laugier. 1995. “Eichhornia crassipes Production on Petroliferous Wastewaters: Effects of Salinity.” Bioresource Technology 54: 39–43.10.1016/0960-8524(95)00112-3
- Center, T. D., and M. P. Hill. 2002. “Field Efficacy and Predicted Host Range of the Pickerelweed Borer, Bellura densa, a Potential Biological Control Agent of Water Hyacinth.” BioControl 47 (2): 231–243.10.1023/A:1014579406894
- Center, T. D., and N. R. Spencer. 1981. “The Phenology and Growth of Water hyacinth (Eichhornia crassipes (Mart.) Solms) in a Eutrophic North-Central Florida Lake.” Aquatic Botany 10: 1–32.10.1016/0304-3770(81)90002-4
- Center, T. D., M. P. Hill, H. Cordo, and M. H. Julien. 2002. “Waterhyacinth.” In Biological Control of Invasive Plants in the Eastern United States, edited by R. G. van Driesche, S. Lyon, B. Blossey, M. S. Hoddle and R. Reardon, 41–64. Morgantown: USDA Forest Service.
- Center, T. D., T. K. Van, F. Allen Dray, S. J. Franks, M. T. Rebelo, P. D. Pratt, and M. B. Rayamajhi. 2005. “Herbivory Alters Competitive Interactions between Two Invasive Aquatic Plants.” Biological Control 33: 173–185.10.1016/j.biocontrol.2005.02.005
- Charudattan, R. 1996. “Pathogens for Biological Control of Water hyacinth.” In Strategies for Water Hyacinth Control, edited by R. Charudattan, R. Labrada, T. D. Center, and C. Kelly-Begazo, 189–196. Rome: Food and Agriculture Organization.
- Charudattan, R. 2001. “Biological Control of Water Hyacinth by using Pathogens: Opportunities, Challenges, and Recent Developments.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center & D. Jianqing, 21-28. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Chikwenhere, G. P., and G. Phiri. 1999. “History of Water Hyacinth and its Control Efforts on Lake Chivero in Zimbabwe.” In Proceedings of the First IOBC Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, edited by M. P. Hill, M. H. Julien, and T. D. Center, 91–97. Pretoria: Plant Protection Research Institute.
- Cilliers, C. J. 1991. “Biological Control of Water Hyacinth, Eichhornia crassipes (Pontederiaceae), in South Africa.” Agriculture, Ecosystems & Environment 37: 207–217.10.1016/0167-8809(91)90149-R
- Coetzee, J. A., and M. P. Hill. 2012. “The Role of Eutrophication in the Biological Control of Water Hyacinth, Eichhornia crassipes, in South Africa.” BioControl 57: 247–261.10.1007/s10526-011-9426-y
- Coetzee, J. A., T. D. Center, M. J. Byrne, and M. P. Hill. 2005. “Impact of the Biological Control Agent Eccritotarsus catarinensis, a sap-Feeding Mirid, on the Competitive Performance of Water Hyacinth, Eichhornia crassipes.” Biological Control 32: 90–96.10.1016/j.biocontrol.2004.08.001
- Coetzee, J. A., M. P. Hill, M. J. Byrne, and A. Bownes. 2011. “A Review of the Biological Control Programmes on Eichhornia crassipes (C. Mart.) Solms (Pontederiaceae), Salvinia molesta DS Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides Lam. (Azollaceae) in South Africa.” African Entomology 19 (2): 451–468.10.4001/003.019.0202
- Coetzee, J. A., R. W. Jones, and M. P. Hill. 2014. “Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces Benthic Macroinvertebrate Diversity in a Protected Subtropical Lake in South Africa.” Biodiversity & Conservation 23: 1319–1330.10.1007/s10531-014-0667-9
- Cordo, H. A. 1999. “New Agents for Biological Control of Water Hyacinth.” In Proceedings of the First IOBC Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, edited by M. P. Hill, M. H. Julien, and T. D. Center, 68-74. Pretoria: Plant Protection Research Institute.
- Cronk, J. K., and M. S. Fennessy. 2001. Wetland Plants: Biology and Ecology. Boca Raton: CRC Press.10.1201/9781420032925
- Dagno, K. 2006. “Évaluation des microorganismes fongiques en tant qu’agents de lutte biologique contre Eichhornia crassipes (Martius) Solms-Laubach dans le bassin du fleuve Niger au Mali.” DEA doc. Sci. agron. Gembloux, Belgique: Gembloux Agricultural University – FUSAGx.
- Dagno, K. 2011. Selection, Efficacy, Ecological Characterization and Formulation of Fungal Control Agents against Water Hyacinth (Eichhornia crassipes (Martius) Solms-Laubach) in Mali. PhD Diss.: University de Liege, Gembloux Agro-BioTech.
- DAISIE. 2008. Delivering Alien Invasive Species Inventories for Europe. DAISIE (online). www.europe-aliens.org
- Datta, S. C., and A. K. Banerjee. 1978. “Useful Weeds of West Bengal Rice Fields.” Economic Botany 32: 297–310.10.1007/BF02864704
- De Groote, H., O. Ajuonu, S. Attignon, R. Djessou, and P. Neuenschwander. 2003. “Economic Impact of Biological Control of Water Hyacinth in Southern Benin.” Ecological Economics 45: 105–117.10.1016/S0921-8009(03)00006-5
- Djeddour, D.H., R.H. Shaw, H.C. Evans, R.A. Tanner, D. Kurose, N. Takahashi, and M. Seier. 2008. “Could Fallopia japonica be the First Target for Classical Weed Biocontrol in Europe.” In Proceedings of the XII International Symposium on Biological Control of Weeds, edited by M.H. Julien, R. Sforza, M.C. Bon, H.C. Evans, P.E. Hatcher, H.L. Hinz, and B.G. Rector, 463-469. Wallingford: CABI.
- Duarte, L. 2017. Water Hyacinth Invasion on Guadiana River. Some Number, Facts and Thoughts. Steemit. Biology-Blogs. Accessed June 15, 2017. https://steemit.com/biology/@liliana.duarte/water-hyacinth-invasion-on-guadiana-river-some-numbers-facts-and-thoughts)
- Eckenwalder, J. E., and S. C. H. Barrett. 1986. “Phylogenetic Systematics of Pontederiaceae.” Systematic Botany 11: 373–391.10.2307/2419074
- Edwards, D., and C. J. Musil. 1975. “Eichhornia crassipes in South Africa-a General Review.” Journal of the Limnological Society of Southern Africa 1: 23–27.10.1080/03779688.1975.9632904
- El-Morsy, E. M. 2004. “Evaluation of Microfungi for the Biological Control of Water Hyacinth in Egypt.” Fungal Diversity 16: 35–51.
- EPPO. 2008. “EPPO/CoE Workshop: How to Manage Invasive Alien Plants? The Case Studies of Eichhornia crassipes and Eichhornia azurea” https://archives.eppo.int/MEETINGS/2008_conferences/eicchornia_workshop.htm.
- EPPO. 2009. “PM 9/8(1): Eichhornia crassipes.” EPPO Bulletin 39: 460–464.
- EU. 2014, November 4. “Regulation (EU) No. 1143/2014 of the European Parliament and of the Council on the Prevention and Management of the Introduction and Spread of Invasive Alien Species.” Official Journal of the European Union L 317: 35–55.
- Evans, H. C., and R. H. Reeder. 2001. “Fungi Associated with Eichhornia crassipes (Water hyacinth) in the Upper Amazon Basin and Prospects for their Use in Biological Control.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 62–70. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Fagúndez, G. A., P. D. Reinoso, and P. G. Aceñolaza. 2016. “Caracterización y fenología de especies de interés apícola en el departamento Diamante (Entre Ríos, Argentina). [Characterization and Phenology of Apicultural Species in the Diamante department (Entre Ríos, Argentina)].” Boletín de la Sociedad Argentina de Botánica 51: 243–267.
- Figueiredo, J., C. Duarte, I. Moreira, and S. Agusti. 1984. “As infestantes aquáticas nos sistemas de irrigação e drenagem do Ribatejo. [Aquatic Weeds in Ribatejo Irrigation and Drainage Systems].” Recursos Hídricos 5: 5–14.
- Forno, I. W. 1983. “Life History and Biology of a Water Hyacinth moth, Argyractis subornata (Lepidoptera: Pyralidae, Nymphulinae).” Annals of the Entomological Society of America 76 (4): 624–627.10.1093/aesa/76.4.624
- Freeman, T. E., R. Charudattan, and K. E. Conway. 1981. Biological Control of Aquatic Plants with Pathogenic Fungi. Technical Report. Vicksburg: U. S. Army Corps of Engineers, Waterways Experiment Station.
- Fried, G. 2017. Guide des plantes invasives. Nouvelle édition [Guide of invasive plants. 2nd ed.]. Paris: Belin.
- GBIF.org. 2017, July 13. GBIF Occurrence Download https://doi.org/10.15468/dl.ple8ca
- Georges, N., and N. Pax. 2002. “Pistia stratiotes L. et Eichhornia crassipes (Mart) Solms, deux nouvelles hydrophytes dans la vallée de la Moselle.” [Pistia stratiotes L. and Eichornia crassipes (Mart) Solms, two new hydrophyts in the Moselle valley] Willemetia 28: 3–4.
- GIC. Grupo de Investigación en Biología de la Conservación de la Universidad de Extremadura. 2006. “Informe sobre Distribución y Biología Reproductora del Jacinto de Agua en el Guadiana” 12 vols. Confederación Hidrográfica del Guadiana. Ministerio de Ambiente, Badajoz, España. 12 vols. Vol. 1 (135 pp), vol. 2 (247 pp), vol. 3 (80 pp), vol. 4 (342 pp), vol. 5 (394 pp), vol. 6 (102 pp), vol. 7 (49 pp), vol. 8 (127 pp), vol. 9 (87 pp), vol. 10 (558 pp), vol. 11 (121 pp), vol. 12 (386 pp).
- Gichuki, J., R. Omondi, P. Boera, T. Okorut, A. S. Matano, T. Jembe, and A. Ofulla. 2012. “Water hyacinth Eichhornia crassipes (Mart.) Solms-Laubach Dynamics and Succession in the Nyanza Gulf of Lake Victoria (East Africa): Implications for Water Quality and Biodiversity Conservation.” The Scientific World Journal. Article ID 106429, 10 pages. doi:10.1100/2012/106429.
- Gopal, B. 1987. Water Hyacinth. Amsterdam: Elsevier Science Publishers.
- Gopal, B., and U. Goel. 1993. “Competition and Allelopathy in Aquatic Plant Communities.” The Botanical Review 59: 155–210.10.1007/BF02856599
- Gossett, D. R., and W. E. Norris. 1971. “Relationship between Nutrient Availability and Content of Nitrogen and Phosphorus in Tissues of the Aquatic Macrophyte, Eichhornia crassipes (Mart.) Solms.” Hydrobiologia 38: 15–28.10.1007/BF00036789
- Gu, R. 1991. “Water hyacinth.” In Chinese Agricultural Encyclopedia, edited by S. Jin, 513. Beijing: Chinese Agricultural Press.
- Guerreiro, A. R. 1976. “O Jacinto aquatico (Eichhornia crassipes (Mart) Solms) em Portugal.” [Water hyacinth (Eichhornia crassipes (Mart) Solms) in Portugal].” In II Simposio Nacional de Herbologia, edited by SCAP-SPFF. Oeiras: Sociedade de Ciências Agrárias de Portugal, Sociedade Portuguesa de Fitiatria e Fitofarmacologia.
- Guna, V. K., M. Ilangovan, A. M. Gangadharaiah, and N. Reddy. 2017. “Water Hyacinth: A Unique Source for Sustainable Materials and Products.” ACS Sustainable Chemistry & Engineering 5: 4478–4490.10.1021/acssuschemeng.7b00051
- Gutiérrez, E., R. Huerto, P. Saldaña, and F. Arreguín. 1996. “Strategies for Water Hyacinth (Eichhornia crassipes) Control in Mexico.” Hydrobiologia 340: 181–185.10.1007/BF00012752
- Gutter, P. 2001. “Environment and Law in Burma.” Legal Issues on Burma Journal 9 (1): 69.
- Haller, W. T., and D. L. Sutton. 1973. “Effect of pH and High Phosphorus Concentrations on Growth of Water Hyacinth.” Hyacinth Control Journal 11: 59–61.
- Hansen, K. L., E. G. Ruby, and R. L. Thompson. 1971. “Trophic Relationships in the Water Hyacinth Community.” Quarterly Journal of the Florida Academy of Sciences 34: 107–113.
- Harley, K. L. S. 1990. “The Role of Biological Control in the Management of Water Hyacinth, Eichhornia crassipes.” Biocontrol News and Information 11: 11–22.
- Heard, T. A., R. Zonneveld, and G. Fichera. 2014. “Megamelus scutellaris (Hemiptera: Delphacidae), a Biocontrol Agent of Water Hyacinth, is not Sufficiently Specific for Release in Australia.” Biocontrol Science and Technology 24: 554–560.10.1080/09583157.2013.876616
- Henderson, L., and C.J. Cilliers. 2002. Invasive Aquatic Plants. Plant Protection Research Institute Handbook No. 16, Agricultural Research Council, Pretoria, South Africa.
- Hernández, M. C., J. S., and G. Cabrera Walsh. 2011. “Biology and Host Preference of the planthopper Taosa longula (Hemiptera: Dictyopharidae), a Candidate for Biocontrol of Water Hyacinth.” Biocontrol Science and Technology 21: 1079–1090.10.1080/09583157.2011.604714
- Hill, M. P. 2003. “The Impact and Control of Alien Aquatic Vegetation in South African Aquatic Ecosystems.” African Journal of Aquatic Science 28: 19–24.
- Hill, M. P., and J. A. Coetzee. 2008. “Integrated Control of Water Hyacinth in Africa.” EPPO Bulletin 38: 452–457.10.1111/epp.2008.38.issue-3
- Hill, M. P., and J. A. Coetzee. 2017. “The Biological Control of Aquatic Weeds in South Africa: Current Status and Future Challenges.” Bothalia-African Biodiversity & Conservation 47: 1–12.
- Hill, M. P., and M. H. Julien. 2004. “The Transfer of Appropriate Technology; Key to the Successful Biological Control of Five Aquatic Weeds in Africa.” In XI International Symposium on Biological Control of Weeds, edited by M. Cullen, D. T. Briese, D. J. Kriticos, W. M. Lonsdale, L. Morin and J. K. Scott, 370–374. Canberra: CSIRO Entomology.
- Hill, M. P., and T. Olckers. 2001. “Biological Control Initiatives Against Water Hyacinth in South Africa: Constraining Factors, Success and New Courses of Action.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 33-38. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Hill, G., R. Day, G. Phiri, C. Lwanda, F. Njaya, S. Chimatiro, and M.P. Hill. 1999. Water Hyacinth Biological Control In the Shire River, Malawi. In Proceedings of the First IOBC Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, edited by M.P. Hill, M.H. Julien, and T.D. Center, 62-66. Pretoria: Plant Protection Research Institute.
- Hussner, A., and R. Lösch. 2005. “Alien Aquatic Plants in a Thermally Abnormal River and their Assembly to Neophyte-Dominated Macrophyte Stands (River Erft, Northrhine-Westphalia).” Limnologica-Ecology and Management of Inland Waters 35: 18–30.10.1016/j.limno.2005.01.001
- Hussner, A., S. Nehring, and S. Hilt. 2014. “From First Reports to Successful Control: A Plea for Improved Management of Alien Aquatic Plant Species in Germany.” Hydrobiologia 737: 321–331.
- Iberite, M., and I. Pelliccioni. 2009. “La flora delle acque interne dell’Agro Pontino (Lazio Meridionale): Indagini preliminari [The Flora of the Internal Waters of Agro Pontino (Southern Lazio): Preliminary Investigations].” Annales Botanici (NS) 9: 155–164.
- Iqbal, I. 2010. The Bengal Delta: Ecology, State and Social Change, 1840–1943. London: Palgrave MacMillan.10.1057/9780230289819
- Jianqing, D., W. Ren, F. Weidong, and Z. Guoliang. 2001. “Water Hyacinth in China: Its Distribution, Problems and Control Status.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 102. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Jones, R. W. 2001. “Integrated Control of Water Hyacinth on the Nseleni/Mposa Rivers and Lake Nsezi in KwaZulu-Natal, South Africa.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 123–129. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Jones, R., and C. J. Cilliers. 1999. “Integrated Control of Water Hyacinth on the Enseleni/Mposa Rivers and Lake Nsezi in KwaZulu Natal, South Africa.” In Proceedings of the First IOBC Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, edited by M. P. Hill, M. H. Julien, and T. D. Center, 160–167. Pretoria: Plant Protection Research Institute.
- Julien, M. H. 2001. “Biological Control of Water Hyacinth with Arthropods: A Review to 2000.” In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 8–20. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Julien, M. H. 2008. “Plant Biology and Other Issues that Relate to the Management of Water Hyacinth: A Global Perspective with Focus on Europe.” EPPO Bulletin 38: 477–486.10.1111/epp.2008.38.issue-3
- Julien, M. H., and M. W. Griffiths. 1998. Biological Control of Weeds: A World Catalogue of Agents and their Target Weeds. 4th ed. Wallingford: CABI Publishing.
- Julien, M. H., K. L. S. Harley, A. D. Wright, C. J. Cilliers, M. P. Hill, T. D. Center, H. A. Cordo, and A. F. Cofrancesco. 1996. “International Co-operation and Linkages in the Management of Water Hyacinth with Emphasis on Biological Control.” In Proceedings of the IX International Symposium on Biological Control of Weeds, edited by V. C. Moran and J. H. Hoffman, 273–282. Stellenbosch: University of Cape Town.
- Julien, M. H., M. W. Griffiths, and A. D. Wright. 1999. Biological Control of Water Hyacinth: The Weevil Neochetina bruchi and N. eichhorniae: Biologies, Host Ranges, and Rearing, Releasing and Monitoring Techniques for Biological Control of Eichhornia crassipes. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Kaplan, Z., J. Danihelka, M. Lepší, P. Lepší, L. Ekrt, J. Chrtek Jr., J. Kocián, et al. 2016. “Distributions of Vascular Plants in the Czech Republic. Part 3.” Preslia 88 (4): 459–544.
- Khanna, S., M. J. Santos, E. L. Hestir, and S. L. Ustin. 2012. “Plant Community Dynamics Relative to the Changing Distribution of A Highly Invasive Species, Eichhornia crassipes: A Remote Sensing Perspective.” Biological Invasions 14: 717–733.10.1007/s10530-011-0112-x
- Kiefer, H. H. 1979. Eriophyid Studies C-17. Science and Education Adminstration, Agricultural Research Service, U.S. Dep. of Agriculture, Issued August 27, 24 pp.
- Klorer, J. 1909. “The Water Hyacinth Problem.” Journal of the Assoc. Eng. Soc 42: 33–48.
- Kriticos, D. J., and S. Brunel. 2016. “Assessing and Managing the Current and Future Pest Risk from Water Hyacinth (Eichhornia crassipes), An Invasive Aquatic Plant Threatening the Environment and Water Security.” PloS One 11: e0120054.10.1371/journal.pone.0120054
- Kusewa, M. T. 2002. Natural Occurrence and Potential of Pathogenic Fungi for Integrated Management of Water Hyacinth, Eichhornia crassipes (Mart.) Solms-Laubach: Pontederiaceae, in Lake Victoria. Unpublished Master’s Thesis in Environmental Studies. School of Environmental Studies, Moi University.
- Laranjeira, C. M., and G. Nadais. 2008. “Eichhornia crassipes Control in the Largest Portuguese Natural Freshwater Lagoon.” EPPO Bulletin 38: 487–495.10.1111/epp.2008.38.issue-3
- Lastrucci, L., and B. Foggi. 2006. “Prima segnalazione di Eichhornia crassipes (Mart.) Solms (Pontederiaceae) per la Toscana [First Record of Eichhornia crassipes (Mart.) Solms (Pontederiaceae) in Tuscany].” Atti Soc toscana di Scienze Naturali, Memorie, Serie B 113: 27–30.
- Madsen, J. D. 1993. “Growth and Biomass Allocation Patterns During Water Hyacinth Mat Development.” Journal of Aquatic Plant Management 31: 134–134.
- Mailu, A. M. 2001. “Preliminary Assessment of the Social, Economic and Environmental Impacts of Water Hyacinth in Lake Victoria Basin and Status of Control.” In Biological and Integrated Control of Waterhyacinth, Eichhornia crassipes. In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, edited by M. H. Julien, M. P. Hill, T. D. Center, and D. Jianqing, 130–139. Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Martin, G. D., and J. A. Coetzee. 2011. “Pet stores, Aquarists and the Internet Trade as Modes of Introduction and Spread of Invasive Macrophytes in South Africa.” Water SA 37: 371–380.
- Martín de Rodrigo, E., R. Morán López, T. Ruiz Téllez, and J. M. Sánchez Guzmán. 2008. “Predicitive Models of the Eichhornia crassipes’ Potential Distribution in the Guadiana River (Spain).” How to Manage Invasive Alien Plants: The Case Studies of Eichhornia crassipes and E. azurea. EPPO/CoE Workshop. París: EPPO. Accessed June 15, 2017. https://archives.eppo.int/MEETINGS/2008_conferences/eichhornia_files/04_martin/martin1.HTM
- Masifwa, W. F., T. Twongo, and P. Denny. 2001. “The Impact of Water Hyacinth, Eichhornia crassipes (Mart) Solms on the Abundance and Diversity of Aquatic Macroinvertebrates along the Shores of Northern Lake Victoria, Uganda.” Hydrobiologia 452: 79–88.10.1023/A:1011923926911
- Masin, R., and S. Scortegagna. 2012. “Flora alloctona del Veneto centromeridionale (province di Padova, Rovigo, Venezia e Vicenza –Veneto – NE Italia) [Alien Flora of Southern and Central Veneto (Provinces of Padua, Rovigo, Venice and Vicenza -Veneto – NE Italy].” Natura Vicentina 15: 5–54.
- Matthews, L. J. 1967. “Seedling Establishment of Water Hyacinth.” International Journal of Pest Management C 13: 7–8.10.1080/05331856709432481
- McVea, C., and C. E. Boyd. 1975. “Effects of Water Hyacinth Cover on Water Chemistry, Phytoplankton, and Fish in Ponds.” Journal of Environmental Quality 4: 375–378.10.2134/jeq1975.00472425000400030020x
- Midgley, J. M., M. P. Hill, and M. H. Villet. 2006. “The Effect of Water Hyacinth, Eichhornia crassipes (Martius) Solms Laubach (Pontederiaceae), on Benthic Biodiversity in Two Impoundments on the New Year’s River, South Africa.” African Journal of Aquatic Science 31: 25–30.10.2989/16085910609503868
- Miller, G. 2013. “The Crazy, Ingenious Plan to Bring Hippopotamus Ranching to America.” Wired, December 20. https://www.wired.com/2013/12/hippopotamus-ranching.
- Ministerio de Agricultura, Alimentación y Medio Ambiente. 2013. “Real Decreto 630/2013, de 2 de agosto, por el que se regula el Catálogo español de especies exóticas invasoras.” Boletín Oficial Del Estado Num. 185, Sec. I. Pág. 56764.
- Mitchell, D. S. 1985. “African Aquatic Weeds and their Management.” In Geobotany Volume 6: The Ecology and Management of African Wetland Vegetation, edited by P. Denny, 177–202. Dordrecht: Dr W. Junk Publishers.10.1007/978-94-009-5504-2
- Morris, M. J., A. R. Wood, and A. Den Breeÿen. 1999. “Plant Pathogens and Biological Control of Weeds in South Africa: A Review of Projects and Progress During the Last Decade.” African Entomology Memoir 1: 129–137.
- Muramoto, S., I. Aoyama, and Y. Oki. 1991. “Effect of Salinity on the Concentration of Some Elements in Water Hyacinth (Eichhornia crassipes) at Critical Levels.” Journal of Environmental Science & Health Part A 26: 205–215.10.1080/10934529109375628
- Navarro, L. A., and G. Phiri. 2000. Water Hyacinth in Africa and the Middle East. A Survey of Problems and Solutions. Ottawa: International Development Research Centre.
- Neiff, J. J., A. P. Neiff, and S. A. L. Casco. 2001. “The Effect of Prolonged Floods on Eichhornia crassipes Growth in Paraná River Floodplain Lakes.” Acta Limnologica Brasiliensia 13: 51–60.
- Njiru, M., J. B. Okeyo-Owuor, M. Muchiri, and I. G. Cowx. 2004. “Shifts in the Food of Nile tilapia, Oreochromis niloticus (L.) in Lake Victoria, Kenya.” African Journal of Ecology 42: 163–170.10.1111/aje.2004.42.issue-3
- O’Hara, J. 1967. “Invertebrates Found in Water Hyacinth Mats.” Quarterly Journal of the Florida Academy of Sciences 30: 73–80.
- Orapa, W., and M. H. Julien. 2001. “Insects Used for Biological Control of the Aquatic Weed Water Hyacinth in Papua New Guinea.” Papua New Guinea Journal of Agriculture, Forestry and Fisheries 44: 49–60.
- Osmond, R., and A. Petroeschevsky. 2013. “Water Hyacinth Control Options.” Accessed August 31, 2017. https://weeds.ala.org.au/WoNS/waterhyacinth/docs/Water_hyacinth_control_options_WEB-ACCESSIBLE_VERSION_FINAL_V2.pdf
- Owens, C. S., and J. D. Madsen. 1995. “Low Temperature Limits of Waterhyacinth.” Journal of Aquatic Plant Management 33: 63–68.
- Padilla, D. K., and S. L. Williams. 2004. “Beyond Ballast Water: Aquarium and Ornamental Trades as Sources of Invasive Species in Aquatic Ecosystems.” Frontiers in Ecology and the Environment 2: 131–138.10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2
- Page, A. R., and K. L. Lacey. 2006. Economic Impact Assessment of Australian Weed Biological Control. Adelaide: CRC for Australian Weed Management.
- Paterson, I. D., R. Mangan, D. A. Downie, J. A. Coetzee, M. P. Hill, A. M. Burke, P. O. Downey, T. J. Henry, and S. G. Compton. 2016. “Two in One: Cryptic Species Discovered in Biological Control Agent Populations using Molecular Data and Crossbreeding Experiments.” Ecology and Evolution 6: 6139–6150.10.1002/ece3.2016.6.issue-17
- Peña Bretón, C., and A. S. de la Cruz. 2014. “Experiencias de control de Eichhornia crassipes en la Comunitat Valenciana [Experiences of Control of Eichhornia crassipes in the Comunitat Valenciana].” Paper presented at the Jornadas sobre Especies Invasoras de ríos y zonas húmedas Generalitat Valenciana. Accessed June 15, 2017. https://bdb.cma.gva.es/webdoc/documento.ashx?id=156535
- Penfound, W. T., and T. T. Earle. 1948. “The Biology of the Water Hyacinth.” Ecological Monographs 18: 447–472.10.2307/1948585
- Perkins, B. D. 1974. “Arthropods that Stress Waterhyacinth.” PANS Pest Articles & News Summaries 20: 304–314.
- Pieterse, A. H. 1978. “The Water Hyacinth (Eichhornia crassipes) – A Review.” Abstracts on Tropical Agriculture 4: 9–42.
- Pieterse, A. H., and K. J. Murphy. 1993. Aquatic Weeds: The Ecology and Management of Nuisance Aquatic Vegetation. Oxford: Oxford University Press.
- PPP-Index. 2017. Pflanzenprotrait Eichhornia crassipes. Pflanzeneinkaufsführer für Europa. https://www.ppp-index.de/pppindex.dll/PPP_SHOWPLANT?ZID=14021&MID=2757&UID=EC3DE6FB9C101899C9ED5C9C11837160AFED1D62105545.
- Pyšek, P., J. Danihelka, J. Sádlo, J. Chrtek Jr., M. Chytrý, V. Jarošík, Z. Kaplan, et al. 2012. “Catalogue of Alien Plants of the Czech Republic: Checklist Update, Taxonomic Diversity and Invasion Patterns.” Preslia 84: 155–255.
- Q-Bank Invasive Plants. 2017. Accessed August 31, 2017. https://www.q-bank.eu/Plants/BioloMICS.aspx?Table=Plants%20-%20Species&Rec=81&Fields=All
- Ray, P., and M. P. Hill. 2012. “Fungi Associated with Eichhornia crassipes in South Africa and their Pathogenicity Under Controlled Conditions.” African Journal of Aquatic Science 37: 323–331.10.2989/16085914.2012.712912
- Ray, P., P. A. K. Sushilkumar, and A. K. Pandey. 2008. “Survey and Selection of Potential Pathogens for Biological Control of Waterhyacinth.” Indian Journal of Weed Science 40: 75–78.
- Reddy, K. R., M. Agami, and J. C. Tucker. 1989. “Influence of Nitrogen Supply Rates on Growth and Nutrient Storage by Water Hyacinth (Eichhornia crassipes) Plants.” Aquatic Botany 36: 33–43.10.1016/0304-3770(89)90089-2
- Reddy, K. R., M. Agami, and J. C. Tucker. 1990. “Influence of Phosphorus on Growth and Nutrient Storage by Water Hyacinth (Eichhornia crassipes (Mart.) Solms) Plants.” Aquatic Botany 37: 355–365.10.1016/0304-3770(90)90021-C
- Rommens, W., J. Maes, N. Dekeza, P. Inghelbrecht, T. Nhiwatiwa, E. Holsters, F. Ollevier, B. Marshall, and L. Brendonck. 2003. “The Impact of Water Hyacinth (Eichhornia crassipes) in A Eutrophic Subtropical Impoundment (Lake Chivero, Zimbabwe). I. Water quality.” Archiv für Hydrobiologie 158: 373–388.10.1127/0003-9136/2003/0158-0373
- Room, P. M., and I. V. S. Fernando. 1992. “Weed Invasions Countered by Biological Control: Salvinia molesta and Eichhornia crassipes in Sri Lanka.” Aquatic Botany 42: 99–107.10.1016/0304-3770(92)90001-Y
- Ruiz Téllez, T., P. Brufao Curiel, J. Blanco Salas, and F. Vázquez Pardo. 2016. “Pasado, presente y futuro de una invasión biológica: Eichhornia crassipes (Mart.) Solms (camalote) en el río Guadiana.[Past, Present and Future of A Biological Invasion: Eichhornia crassipes (Mart.) Solms (Water Hyacinth) in the River Guadiana].” Conservación Vegetal 20: 8–9.
- Ruiz Téllez, T., E. Martín de Rodrigo López, G. Lorenzo Granado, E. Albano Pérez, R. Morán López, and J. M. Sánchez Guzmán. 2008. “The Water Hyacinth, Eichhornia crassipes: An Invasive Plant in the Guadiana River Basin (Spain).” Aquatic Invasions 3: 42–53.
- Sabrosky, C. W. 1974. “Eugaurax setigena (Diptera: Chloropidae), A New Stem Miner in Water Hyacinth.” Florida Entomologist 57: 347–348.10.2307/3493492
- Schmitz, D. C., B. V. Nelson, L. E. Nall, and J. D. Schardt. 1991. “Exotic Aquatic Plants in Florida: A Historical Perspective and Review of The Present Aquatic Plant Regulation Program.” In Proceedings of the symposium on exotic pest plants, edited by T. D. Center, R. F. Doren, R. L. Hofstetter, R. L. Myers and L. D. Whiteaker, 303–326. Miami, FL: US Natl. Park Serv. Tech. Rep. NPS/.
- Scoppola, A., M. Iberite, and A. M. Palazzi. 1986. “Sulla presenza di Spirodela polyrrhiza (L.) Schleid. ed Eichhornia crassipes (Mart.) Solms nelle acque interne dell’Agro Pontino (Lazio).[About the Presence of Spirodela polyrrhiza (L.) Schleid. and Eichhornia crassipes (Mart.) Solms in the Internal Waters of Agro Pontino (Lazio)].” Annali di Botanica (Roma), Studi sul territorio 44: 167–176.
- Seabrook, E. L. 1962. “The Correlation of Mosquito Breeding to Hyacinth Plants.” Hyacinth Control Journal 1: 18–19.
- Shabana, Y. M. 2005. “The Use of Oil Emulsions for Improving the Efficacy of Alternaria Eichhorniae as a Mycoherbicide for Waterhyacinth (Eichhornia crassipes).” Biological Control 32: 78–89.
- Shabana, Y. M., R. Charudattan, and M. A. Elwakil. 1995a. “Identification, Pathogenicity, and Safety of Alternaria eichhorniae from Egypt as a Bioherbicide Agent for Water Hyacinth.” Biological Control 5: 123–135.10.1006/bcon.1995.1015
- Shabana, Y. M., R. Charudattan, and M. A. Elwakil. 1995b. “Evaluation of Alternaria eichhorniae as a Bioherbicide for Water Hyacinth (Eichhornia crassipes) in Greenhouse Trials.” Biological Control 5: 136–144.10.1006/bcon.1995.1016
- Shaw, R. H. 2008. “Weed Biological Control Regulation in Europe: Boring but Important.” In Proceedings of the XII International Symposium on biological control of Weeds, edited by M. H. Julien, R. Sforza, M. C. Bon, H. C. Evans, P. E. Hatcher, H. L. Hinz, and B. G. Rector, 484–488. Wallingford: CABI.
- Shaw, R., U. Schaffner, and E. Marchante. 2016. “The Regulation of Biological Control of Weeds in Europe – An Evolving Landscape.” EPPO Bulletin 46: 254–258.10.1111/epp.12308
- Silveira Guido, A. 1965. Natural Enemies of Weed Plants. Final Report on PL-480 Project S9-CR-1 (Jan. 1962 to 15 Nov. 1965) ( Unpublished report). Montevideo: Universidad de la Republica, Facultad de Agronomia.
- Sindhu, R., P. Binod, A. Pandey, A. Madhavan, J. A. Alphonsa, N. Vivek, E. Gnansounou, E. Castro, and V. Faraco. 2017. “Water Hyacinth A Potential Source for Value Addition: An Overview.” Bioresource Technology 230: 152–162.10.1016/j.biortech.2017.01.035
- Smith, R. J. Jr. 1983. “Weeds of Major Economic Importance in Rice and Yield Losses due to Weed Competition.” In Proceedings of the Conference on Weed Control in Rice, 19–36. Los Banos, Phillipines: International Rice Research Institute.
- Stinca, A., G. D’Auria, and R. Motti. 2012. “Integrazioni alla flora vascolare aliena della Campania (Sud Italia) [Additional Records to the Alien Flora of Campania (South Italy)].” Inform Bot Ital 44: 287–293.
- Tag El Seed, M. 1978. “Effect of pH on the Nature of Competition between Eichhornia crassipes and Pistia stratiotes.” Journal of Aquatic Plant Management 16: 53–57.
- Taylor, S. J., D. A. Downie, and I. D. Paterson. 2011. “Genetic Diversity of Introduced Populations of the Water Hyacinth Biological Control Agent Eccritotarsus catarinensis (Hemiptera: Miridae).” Biological Control 58: 330–336.10.1016/j.biocontrol.2011.05.008
- Tegene, S., T. Hussein, T. Tessema, F. Yirefu, B. Carmen, and M. Gossmann. 2012. “Exploration of Fungal Pathogens Associated with Water Hyacinth (Eichhornia crassipes (Mart.) Solms-Laubach) in Ethiopia.” African Journal of Agricultural Research 7: 11–18.
- Thomaz, S. M., and L. M. Bini. 1999. “A expansão das macrófitas aquáticas e implicações para o manejo de reservatórios: um estudo na represa de Itaipu. [The Expansion of Aquatic Macrophytes and Implications for Reservoir Management: A Study at the Itaipu Dam].” In Ecologia de reservatórios: estrutura, função e aspectos sociais, edited by R. Henry, 597–626. Botucatu: FUNDIBIO.
- Tipping, P. W., A. Sosa, E. N. Pokorny, J. Foley, D. C. Schmitz, J. S. Lane, L. Rodgers, et al. 2014. “Release and Establishment of Megamelus scutellaris (Hemiptera: Delphacidae) on Water Hyacinth in Florida.” Florida Entomologist 97: 804–806.10.1653/024.097.0264
- Tison, J.-M., and B. de Foucault. 2014. Flora Gallica. Flore de France. Mèze: Biotope.
- Ueki, K., and Y. Oki. 1979. “Seed Production and Germination of Eichhornia crassipes in Japan.” In Proceedings of the Asian-Pacific Weeds Science Society Conference, 257–260. Asian-Pacific Weed Science Society.
- Ultsch, G. R., and D. S. Anthony. 1973. “The Role of the Aquatic Exchange of Carbon Dioxide in the Ecology of the Water Hyacinth (Eichhornia crassipes).” Florida Scientist 36: 16–22.
- Verloove, F. 2006. “Catalogue of Neophytes in Belgium (1800-2005).” Scripta Botanica Belgica 39: 1–89.
- Villamagna, A. M., and B. R. Murphy. 2010. “Ecological and Socio-Economic Impacts of Invasive Water Hyacinth (Eichhornia crassipes): A Review.” Freshwater Biology 55: 282–298.10.1111/fwb.2010.55.issue-2
- Vollenweider, R. A., and J. Kerekes. 1982. Eutrophication of Waters. Monitoring, Assessment and Control. Paris: Organization for Economic Co-Operation and Development (OECD).
- Whitton, B. A., A. Aziz, B. Kawecka, and J. A. Rother. 1988. “Ecology of Deepwater Rice-Fields in Bangladesh 3. Associated Algae and Macrophytes.” Hydrobiologia 169: 31–42.10.1007/BF00007931
- Winston, R. L., M. Schwarzländer, H. L. Hinz, M. D. Day, M. J. W. Cock, and M. H. Julien, eds. 2014. Biological Control of Weeds: A World Catalogue of Agents and their Target Weeds. 5th ed. FHTET-2014-04. Morgantown: USDA Forest Service.
- Wise, R. M., B. W. Van Wilgen, M. P. Hill, F. Sculthess, D. Tweddle, A. Chabi-Olay, and H. G. Zimmerman. 2007. The Economic Impact and Appropriate Management of Selected Invasive Alien Species on the African Continent. Global Invasive Species Programme CSIR report no. CSIR\NRE\RBSD\ER\2007\0044\C.
- Wright, A. D., and M. F. Purcell. 1995. “Eichhornia crassipes (Mart.) Solms-Laubach.” The Biology of Australian Weeds 1: 111–121.
- Wu, K., B. Gao, J. Su, X. Peng, X. Zhang, J. Fu, S. Peng, and P. K. Chu. 2016. “Large and Porous Carbon Sheets Derived from Water Hyacinth for High-Performance Supercapacitors.” RSC Advances 6: 29996–30003.10.1039/C5RA25098F
- Xie, Y., and D. Yu. 2003. “The Significance of Lateral Roots in Phosphorus (P) Acquisition of Water Hyacinth (Eichhornia crassipes).” Aquatic Botany 75: 311–321.10.1016/S0304-3770(03)00003-2
- Yan, S.-H., W. Song, and J.-Y. Guo. 2017. “Advances in Management and Utilization of Invasive Water Hyacinth (Eichhornia crassipes) in Aquatic Ecosystems–A Review.” Critical Reviews in Biotechnology 37: 218–228.10.3109/07388551.2015.1132406
- Yin, T., X. Zhang, X. Liu, and C. Wang. 2017. “Resource Recovery of Eichhornia crassipes as Oil Superabsorbent.” Marine Pollution Bulletin 118: 267–274.10.1016/j.marpolbul.2017.01.064

